Final ID: 4158676
ALPACAR Phase 2 Trial of Zerlasiran: Multiple doses of a Small-Interfering RNA Targeting Lipoprotein(a) with 60 weeks Folow Up.
OBJECTIVE. To evaluate the effects of zerlasiran on circulating levels of Lp(a) after multiple doses over a 60 week period.
DESIGN. A multicenter study in patients with stable ASCVD and serum concentrations of Lp(a) ≥125 nmol/L, conducted at 26 research sites in Australia, the Czech Republic, Denmark, Netherlands, South Africa, Slovakia, and United Kingdom between January 3, 2023, and April 27, 2023, with last follow up on July 1, 2024.
INTERVENTIONS Participants were randomized to receive a subcutaneous dose of placebo Q16 weeks for 3 doses (n= 23), placebo every 24 weeks for 2 doses (n=24), or zerlasiran 300 mg every 16 weeks for 3 doses (n=42), 300 mg Q24 weeks for 2 doses (n=44), or 450 mg Q24 weeks for 2 doses (n=45).
MAIN OUTCOME MEASURES. The primary outcome was the time-averaged percent change in Lp(a) molar concentration from baseline to 36 weeks (long term follow to 60 weeks).
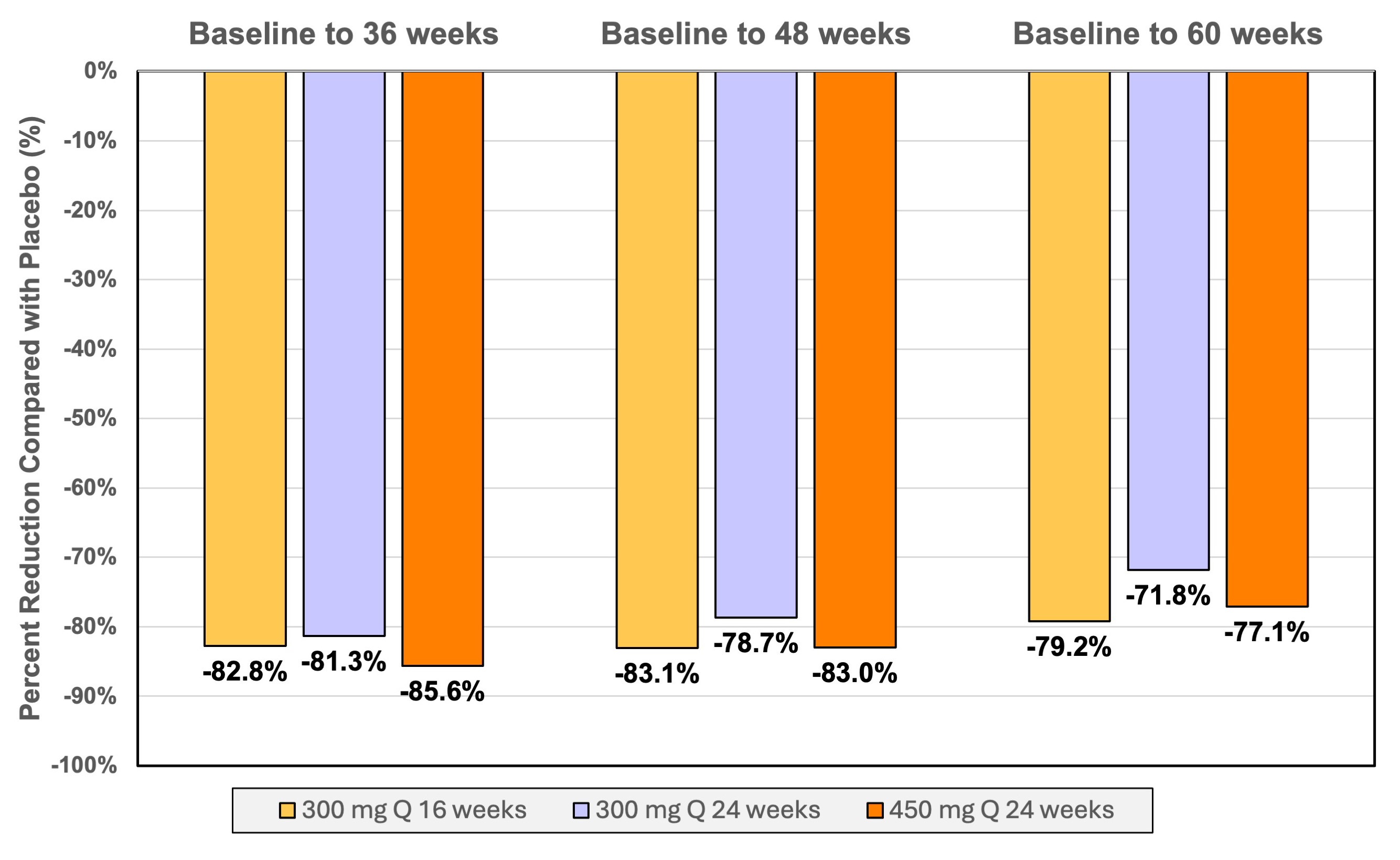
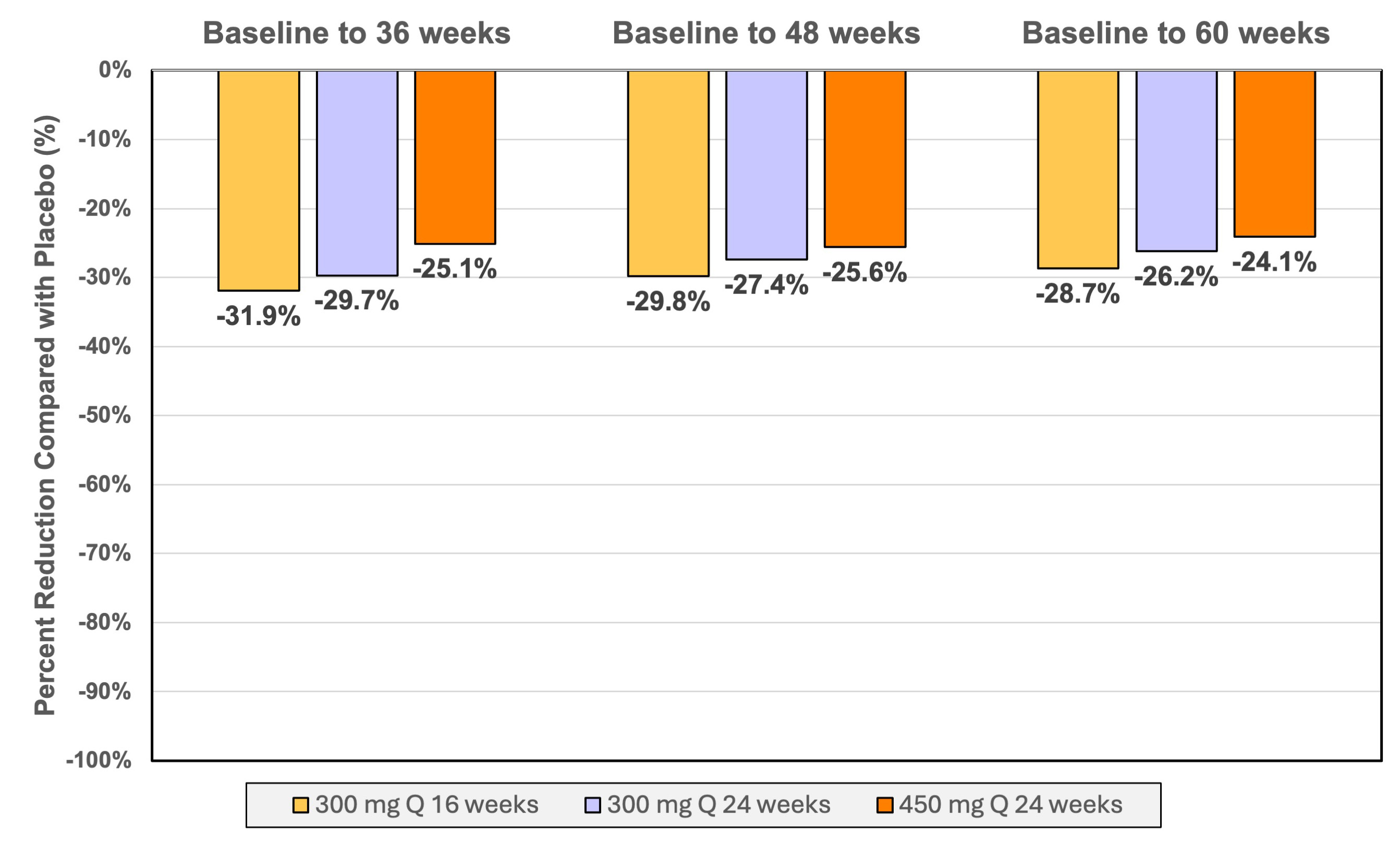
RESULTS. Among 178 patients, mean (SD) age 63.7 (9.4) years; 46 (25.8%) women, with a median baseline Lp(a) molar concentration of 213 (IQR, 177, 282) nmol/L. The placebo-adjusted, mean (95% CI) time-averaged percent change in Lp(a) concentration from baseline to week 36 was -82.8% (-88.2, -77.4%), -81.3% (-86.7, -76.0%)and -85.6% (-90.9, -80.3%) for the groups receiving 300 mg every 16 weeks, 300 mg every 24 weeks, and 450 mg every 24 weeks respectively. Median percentage reductions in Lp(a) greater than 90% were observed for all doses at week 36. The placebo-adjusted mean (95% CI) time-averaged percent change in LDL-C from baseline to week 36 was -31.9% (-54.1, -9.7%), -29.7% (-51.6, -7.8%), and -25.1% (-46.9, -3.3%) for the 300 mg every 16 weeks, 300 mg every 24 weeks, and 450 mg every 24 weeks groups, respectively. Near maximal time-averaged reductions in Lp(a) persisted from baseline to 60 weeks. The most common adverse effects were mild injection site reactions. There were 20 serious adverse events in 17 patients, all unrelated to study drug.
CONCLUSIONS. Zerlasiran was well tolerated and produced greater than 80% reductions in time-averaged Lp(a) concentrations and 25.1 to 31.9% time-averaged reduction in LDL-C during 36 weeks of treatment.
- Nissen, Steven ( Cleveland Clinic , Cleveland , Ohio , United States )
- Dorresteijn, Jannick ( University Medical Center Utrecht , Utrecht , Netherlands )
- Fok, Henry ( Silence Therapeutics , London , United Kingdom )
- Rider, David ( SILENCE THERAPEUTICS GMBH , Berlin , Germany )
- Romano, Steve ( Silence Therapeutics , London , United Kingdom )
- Wolski, Kathy ( Cleveland Clinic , Cleveland , Ohio , United States )
- Rambaran, Curtis ( Silence Therapeutics , Baski Ridge , New Jersey , United States )
- Wang, Qiuqing ( Cleveland Clinic , Cleveland , Ohio , United States )
- Nicholls, Stephen ( Victorian Heart Hospital , Clayton , Victoria , Australia )
- Navar, Ann Marie ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Ray, Kausik ( IMPERIAL COLLEGE LONDON , London , United Kingdom )
- Schwartz, Gregory ( VA EASTERN COLORADO HEALTHCARE SYST , Aurora , Colorado , United States )
- Szarek, Michael ( UNIVERSITY OF COLORADO , Brooklyn , New York , United States )
- Stroes, Erik ( AMC , Amsterdam , Netherlands )
- Troquay, Roland ( VieCuri Med Ctr , Northern Limburg , France )
Meeting Info:
Session Info:
New Targets and New Treatments: Advances in Lipid Therapeutics
Monday, 11/18/2024 , 01:30PM - 02:45PM
Late-Breaking Science
More abstracts on this topic:
Djousse Luc, Leesch Tharen, Pena David, Gaziano Michael, Ward Rachel, Wellman Helen, Yel Nedim, Santos Abigail, Delgrande Jen, Fink Abigail, Colson Kristin, Pan Eddie
A Randomized Phase 2 Trial of Muvalaplin: An Oral Disrupter of the Assembly of Lipoprotein(a) ParticlesNicholls Stephen, Ni Wei, Rhodes Grace, Nissen Steven, Navar Ann Marie, Michael Laura, Krege John
More abstracts from these authors:
Nicholls Stephen, Ni Wei, Rhodes Grace, Nissen Steven, Navar Ann Marie, Michael Laura, Krege John
Differential Cardiovascular Impact of ω-3 Fatty Acid in Patients at High Cardiovascular Risk in Asians versus non-Asians: Sub-analysis of the STRENGTH Randomized Clinical TrialWang Tom Kai Ming, Nicholls Stephen, St John Julie, Wolski Kathy, Nissen Steven