Final ID: MDP1598
Cardiovascular Effectiveness of Glucagon-like Peptide-1 Receptor Agonists and Empagliflozin Combination Therapy in Adults with Type 2 Diabetes
Abstract Body (Do not enter title and authors here): Introduction/Background: Both empagliflozin and glucagon-like peptide-1 receptor agonists (GLP-1RA) have demonstrated cardiovascular (CV) benefits in clinical trials. However, few trials examined the combination of both therapies and evidence is scarce on whether the addition of empagliflozin to GLP-1RA has further CV benefits.
Aim/Goal: We evaluated the risk of CV outcomes in patients with type 2 diabetes (T2D) initiating baseline GLP-1RA therapy that added empagliflozin compared to those that added sulfonylureas (SU).
Methods: Using Medicare, Optum, and Marketscan data (2014-22), we identified patients with T2D aged ≥18 years initiating GLP-1RA that subsequently added either empagliflozin or SU. Follow-up began on the date of additional therapy and continued until discontinuation of either drug, a gap in insurance coverage, death, or study end. After propensity score standardized mortality ratio weighting adjusting for 98 covariates, we estimated hazard ratios (HR) and rate differences (RD) per 1,000 person-years evaluating a composite of myocardial infarction (MI) or stroke, hospitalization for heart failure (HHF), or all-cause death in the overall population and in patients with history of CV disease.
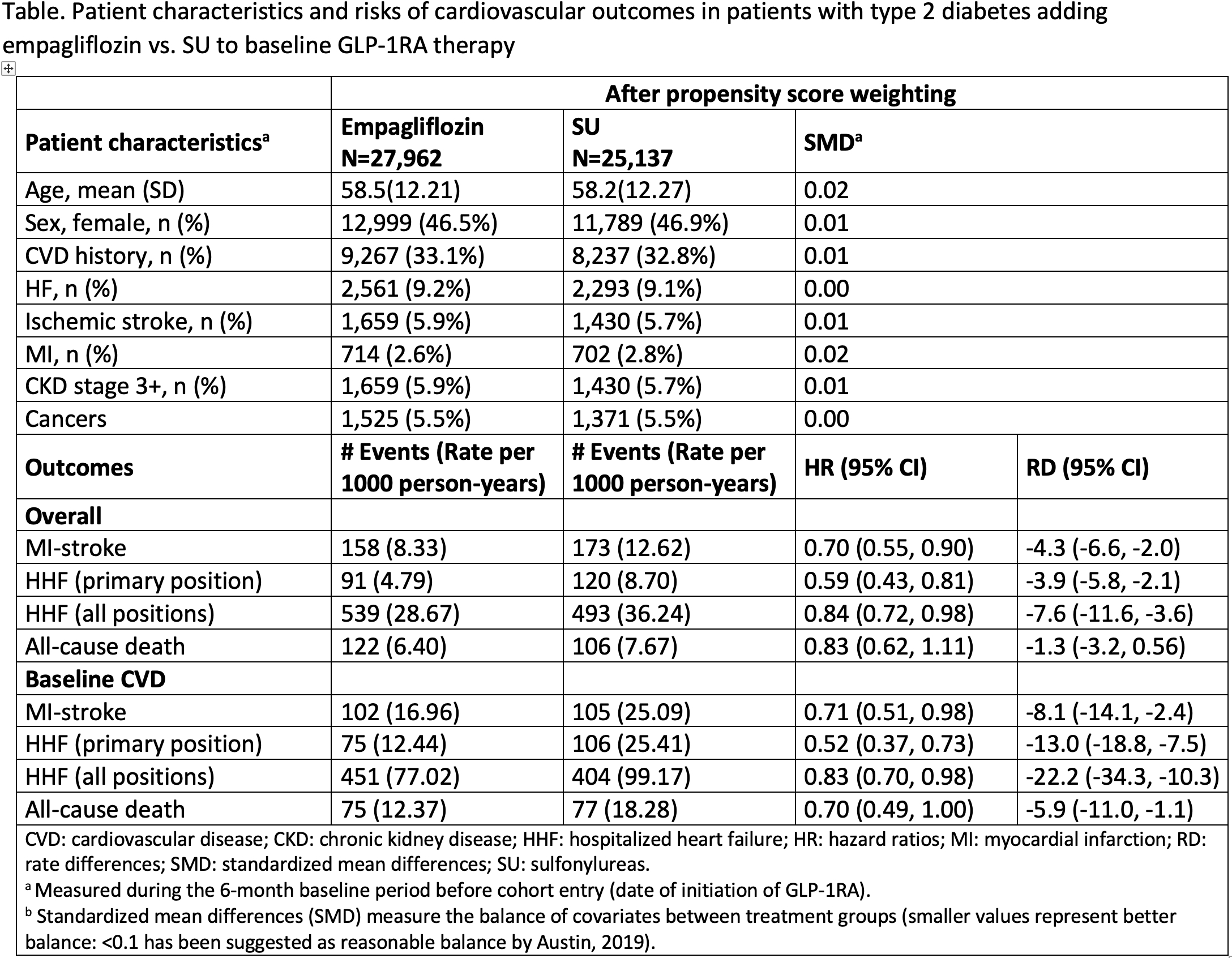
Results: We identified 27,962 patients initiating GLP-1RA + empagliflozin vs 25,137 patients initiating GLP-1RA + SU. After weighting, relative to patients initiating GLP-1RA + SU, those initiating GLP-1RA + empagliflozin had lower risks of MI-stroke [HR 0.70 (0.55, 0.90); RD: -4.3 (-6.6, -2.0)], HHF in the primary hospital discharge position [HR: 0.59 (0.43, 0.81); RD: -3.9 (-5.8, -2.1)]; HHF in any discharge positions [HR: 0.84 (0.72, 0.98); RD: -7.6 (-11.6, -3.6)], and all-cause death [HR: 0.83 (0.62, 1.11); RD: -1.3 (-3.2, 0.56)]. Findings were similar but with larger RDs in patients with baseline CV disease (Table).
Conclusion: In US clinical practice, patients with T2D initiating GLP-1RA that added empagliflozin had lower risks of MI-stroke, HHF, and death compared to adding SU, which suggests an additive CV benefit when combining GLP-1RA and empagliflozin therapies.
Aim/Goal: We evaluated the risk of CV outcomes in patients with type 2 diabetes (T2D) initiating baseline GLP-1RA therapy that added empagliflozin compared to those that added sulfonylureas (SU).
Methods: Using Medicare, Optum, and Marketscan data (2014-22), we identified patients with T2D aged ≥18 years initiating GLP-1RA that subsequently added either empagliflozin or SU. Follow-up began on the date of additional therapy and continued until discontinuation of either drug, a gap in insurance coverage, death, or study end. After propensity score standardized mortality ratio weighting adjusting for 98 covariates, we estimated hazard ratios (HR) and rate differences (RD) per 1,000 person-years evaluating a composite of myocardial infarction (MI) or stroke, hospitalization for heart failure (HHF), or all-cause death in the overall population and in patients with history of CV disease.
Results: We identified 27,962 patients initiating GLP-1RA + empagliflozin vs 25,137 patients initiating GLP-1RA + SU. After weighting, relative to patients initiating GLP-1RA + SU, those initiating GLP-1RA + empagliflozin had lower risks of MI-stroke [HR 0.70 (0.55, 0.90); RD: -4.3 (-6.6, -2.0)], HHF in the primary hospital discharge position [HR: 0.59 (0.43, 0.81); RD: -3.9 (-5.8, -2.1)]; HHF in any discharge positions [HR: 0.84 (0.72, 0.98); RD: -7.6 (-11.6, -3.6)], and all-cause death [HR: 0.83 (0.62, 1.11); RD: -1.3 (-3.2, 0.56)]. Findings were similar but with larger RDs in patients with baseline CV disease (Table).
Conclusion: In US clinical practice, patients with T2D initiating GLP-1RA that added empagliflozin had lower risks of MI-stroke, HHF, and death compared to adding SU, which suggests an additive CV benefit when combining GLP-1RA and empagliflozin therapies.
More abstracts on this topic:
Evaluating Tenecteplase Versus Alteplase in Acute Ischemic Stroke Management: Real-World Insights for Clinical Decision-Making
Hong Lucia, Bannoud Makhlouf, Ibelaidene Maya, Woo Daniel, Novak Daniel
Cardiac Electrophysiologic Response to Single-dose AUX-001, a Once-Daily Extended-Release Nicorandil in Development for Chronic-stable Angina in Adult Healthy Volunteers under Fasting and Fed ConditionsTigoer Uwe, Chabowski Dawid, Fonseca Marlene