Final ID: Sa3024
Olezarsen for Hypertriglyceridemia in Patients at High Cardiovascular Risk - A Systematic Review and Meta Analysis
Abstract Body (Do not enter title and authors here): Abstract
Background: Hypertriglyceridemia, a common lipid disorder linked to increased risks of cardiovascular diseases and metabolic syndrome, remains a global health challenge. Traditional treatments, though widely used, often have limited efficacy and adverse effects. Olezarsen, a novel antisense oligonucleotide, targets apolipoprotein C-III and shows promise in significantly reducing triglyceride levels.
Aim: To conduct a meta-analysis comparing Olezarsen with placebo in patients with hypertriglyceridemia.
Methods: We performed an extensive literature search of PubMed, EMBASE, and Cochrane Library databases up to May 2024 to identify studies comparing Olezarsen and placebo in patients with hypertriglyceridemia, irrespective of comorbid cardiovascular disease. Effect estimates were pooled using a random-effects model and reported as risk ratios (RR) for dichotomous outcomes and standard mean differences (SMD) for continuous outcomes, with 95% confidence intervals (CIs) for each.
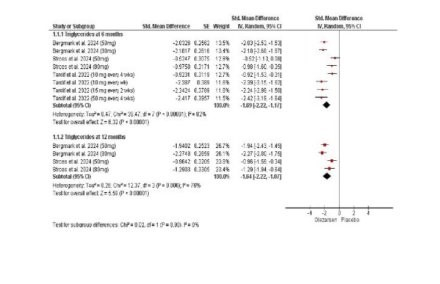
Results: Three randomized controlled trials (RCTs) with a total of 334 participants were included. Olezarsen significantly reduced triglyceride levels at 6 months (SMD: -1.69; 95% CI -2.22 to -1.17; P < 0.00001; Figure) and 12 months (SMD: -1.64; 95% CI -2.22 to -1.07; P < 0.00001; Figure). VLDL levels also decreased at 6 months (SMD: -1.95; 95% CI -2.38 to -1.51; P < 0.00001) and 12 months (SMD: -0.83; 95% CI -1.13 to -0.53; P < 0.00001). Lipid profile improvements included reductions in total cholesterol, non-HDL cholesterol, and apoB levels, with increases in HDL cholesterol and apoA-1. Adverse events were comparable between Olezarsen and placebo, although serious adverse events were significantly higher in the Olezarsen group (RR: 2.55; 95% CI 1.17 to 5.58; p = 0.02).
Conclusion: Olezarsen demonstrates significant efficacy in reducing triglyceride and VLDL levels while improving overall lipid profiles in hypertriglyceridemia patients. Despite higher rates of serious adverse events, its overall safety profile remains acceptable.
Background: Hypertriglyceridemia, a common lipid disorder linked to increased risks of cardiovascular diseases and metabolic syndrome, remains a global health challenge. Traditional treatments, though widely used, often have limited efficacy and adverse effects. Olezarsen, a novel antisense oligonucleotide, targets apolipoprotein C-III and shows promise in significantly reducing triglyceride levels.
Aim: To conduct a meta-analysis comparing Olezarsen with placebo in patients with hypertriglyceridemia.
Methods: We performed an extensive literature search of PubMed, EMBASE, and Cochrane Library databases up to May 2024 to identify studies comparing Olezarsen and placebo in patients with hypertriglyceridemia, irrespective of comorbid cardiovascular disease. Effect estimates were pooled using a random-effects model and reported as risk ratios (RR) for dichotomous outcomes and standard mean differences (SMD) for continuous outcomes, with 95% confidence intervals (CIs) for each.
Results: Three randomized controlled trials (RCTs) with a total of 334 participants were included. Olezarsen significantly reduced triglyceride levels at 6 months (SMD: -1.69; 95% CI -2.22 to -1.17; P < 0.00001; Figure) and 12 months (SMD: -1.64; 95% CI -2.22 to -1.07; P < 0.00001; Figure). VLDL levels also decreased at 6 months (SMD: -1.95; 95% CI -2.38 to -1.51; P < 0.00001) and 12 months (SMD: -0.83; 95% CI -1.13 to -0.53; P < 0.00001). Lipid profile improvements included reductions in total cholesterol, non-HDL cholesterol, and apoB levels, with increases in HDL cholesterol and apoA-1. Adverse events were comparable between Olezarsen and placebo, although serious adverse events were significantly higher in the Olezarsen group (RR: 2.55; 95% CI 1.17 to 5.58; p = 0.02).
Conclusion: Olezarsen demonstrates significant efficacy in reducing triglyceride and VLDL levels while improving overall lipid profiles in hypertriglyceridemia patients. Despite higher rates of serious adverse events, its overall safety profile remains acceptable.
More abstracts on this topic:
A Health Coach-Based Multi-Level Personalized Strategy Lowers LDL-Cholesterol and Enhances Lipid Control in Veterans with Atherosclerotic Cardiovascular Disease – The VA Lipid Optimization Reimagined Quality Improvement Project at VA New York Harbor Healthcare System
Chen Tina, Ingerman Diana, Haley Leah, Salovaara Priscilla, Nicholson Andrew, Illenberger Nicholas, Natarajan Sundar
A drug target Mendelian randomization study of triglyceride lowering therapies for aortic stenosisCiofani Jonathan, Han Daniel, Gill Dipender, Rao Karan, Allahwala Usaid, Bhindi Ravinay