Final ID: Sa1045
Menopausal Status Protects Against Vascular Dysfunction Induced by Breast Cancer Chemotherapy
Abstract Body (Do not enter title and authors here): Introduction: Breast cancer chemotherapy is associated with an increased risk of cardiovascular events, particularly pronounced in postmenopausal women. Vascular damage and docetaxel-caused endothelial dysfunction may underlie these effects, although the role of estrogens and menopausal status remains undefined.
Hypothesis: Estrogens protect premenopausal women from neoadjuvant chemotherapy-induced endothelial dysfunction (NACT regimen: docetaxel, doxorubicin, cyclophosphamide) and its component docetaxel.
Methods: We studied vascular function and its molecular determinants in mammary artery branches from pre- and postmenopausal women undergoing breast cancer surgery, with or without prior NACT. Mechanisms were investigated in bilaterally ovariectomized female C57BL/6J mice treated with docetaxel or placebo.
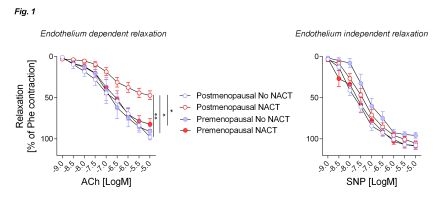
Results: While NACT evoked endothelial dysfunction characterized by the loss of nitric oxide bioavailability in blood vessels from postmenopausal women, premenopausal women were protected from this phenomenon (Fig. 1). Furthermore, premenopausal women’s blood vessels exhibited resilience against NACT-induced vascular oxidative stress. In particular, NOX4 expression was markedly lower in NACT-treated arteries from premenopausal women compared to their postmenopausal counterparts. No differences were seen in the non-NACT groups. At the molecular level, NACT incited lower inhibitory phosphorylation of Thr495 eNOS in premenopausal women's arteries compared to those of postmenopausal women post-NACT. In mice following bilateral ovariectomy, docetaxel caused endothelial dysfunction, but did not perturb acetylcholine-induced vasodilation in non-ovariectomized mice. Docetaxel induced blood pressure only in the group of ovariectomized mice.
Conclusions: NACT-induced vascular dysfunction, often observed in breast cancer survivors, is absent in premenopausal women. This can be partially attributed to the protective role of estrogens, which prevent excessive production of reactive oxygen species and inhibitory eNOS phosphorylation.
Hypothesis: Estrogens protect premenopausal women from neoadjuvant chemotherapy-induced endothelial dysfunction (NACT regimen: docetaxel, doxorubicin, cyclophosphamide) and its component docetaxel.
Methods: We studied vascular function and its molecular determinants in mammary artery branches from pre- and postmenopausal women undergoing breast cancer surgery, with or without prior NACT. Mechanisms were investigated in bilaterally ovariectomized female C57BL/6J mice treated with docetaxel or placebo.
Results: While NACT evoked endothelial dysfunction characterized by the loss of nitric oxide bioavailability in blood vessels from postmenopausal women, premenopausal women were protected from this phenomenon (Fig. 1). Furthermore, premenopausal women’s blood vessels exhibited resilience against NACT-induced vascular oxidative stress. In particular, NOX4 expression was markedly lower in NACT-treated arteries from premenopausal women compared to their postmenopausal counterparts. No differences were seen in the non-NACT groups. At the molecular level, NACT incited lower inhibitory phosphorylation of Thr495 eNOS in premenopausal women's arteries compared to those of postmenopausal women post-NACT. In mice following bilateral ovariectomy, docetaxel caused endothelial dysfunction, but did not perturb acetylcholine-induced vasodilation in non-ovariectomized mice. Docetaxel induced blood pressure only in the group of ovariectomized mice.
Conclusions: NACT-induced vascular dysfunction, often observed in breast cancer survivors, is absent in premenopausal women. This can be partially attributed to the protective role of estrogens, which prevent excessive production of reactive oxygen species and inhibitory eNOS phosphorylation.
More abstracts on this topic:
A Diagnostic Pitfall: Subclavian Stenosis Mimicking Severe Aortic Stenosis on Echocardiography"
Ezaldin Shady, Abdelsalam Mahmoud, Elsayed Omar, Lee Marciano
A Case of Steroid-Refractory Immune-checkpoint-inhibitor Induced Myocarditis Responsive to Mycophenolate and Anti-thymocyte globulinDabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey