Final ID: MDP748
National Estimates of Patient Eligibility for Renal Denervation Therapy Post-FDA Approval
Abstract Body (Do not enter title and authors here): Background
Renal denervation (RDN) has been shown in randomized trials to improve blood pressure compared with a sham procedure. Currently, there are two FDA-approved RDN devices in the United States (US). While nearly half of the US population has hypertension (HTN), the number of patients who may benefit from RDN therapy remains uncertain. In this study, we used a nationally representative dataset to approximate the proportion of patients with HTN who may be eligible for consideration of RDN based on selective criteria.
Methods
All adult patients with HTN who participated in the National Health and Nutrition Examination Survey (NHANES) between the years 2009-2020 were identified. We characterized the proportion of these participants that met eligibility criteria based on 1) the FDA indication, 2) the SCAI 2023 RDN position statement, and 3) enrollment criteria from the RDN on-medication randomized trials. National estimates were obtained utilizing survey weighting from the NHANES multistage probability survey design.
Results
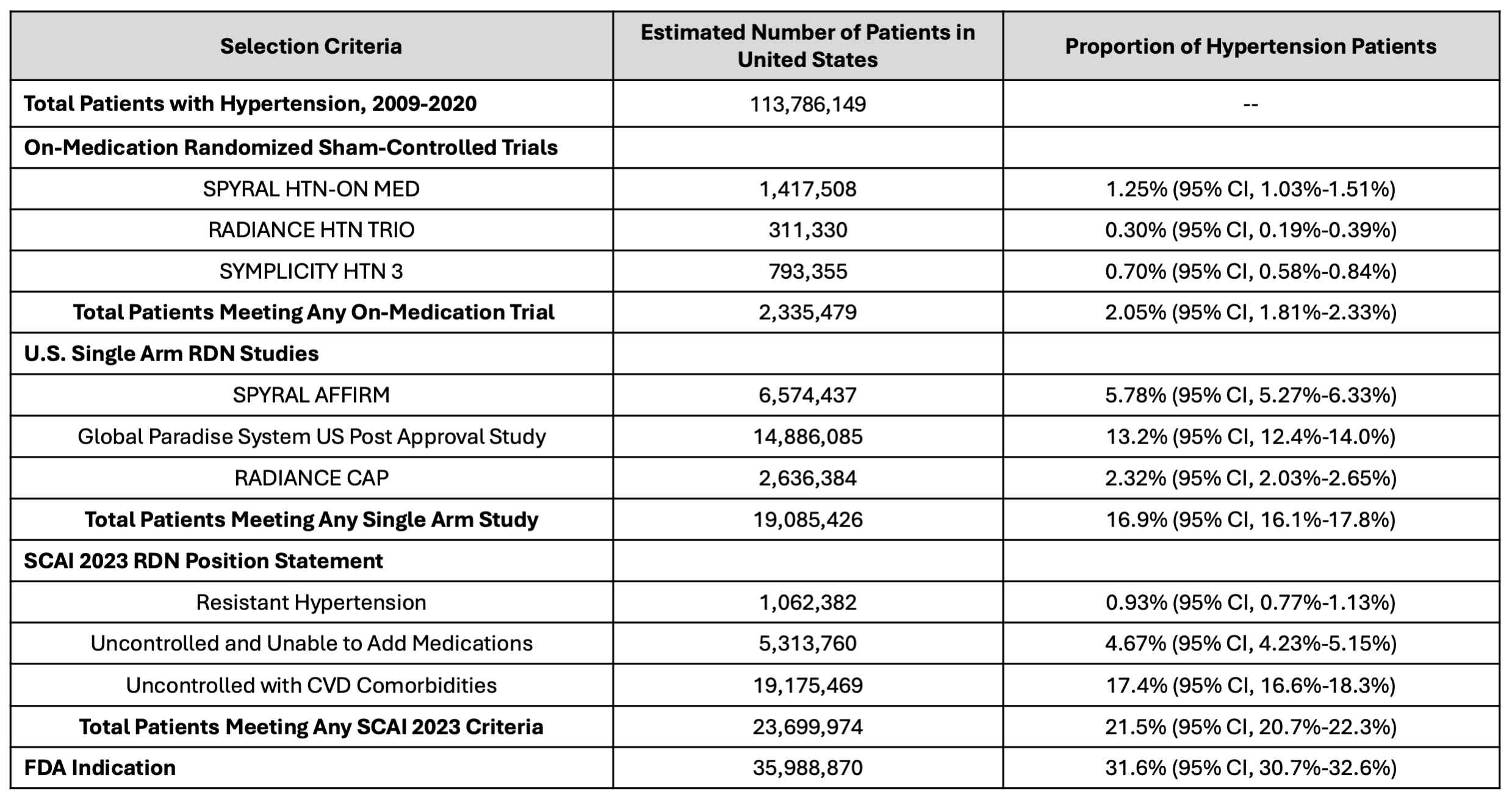
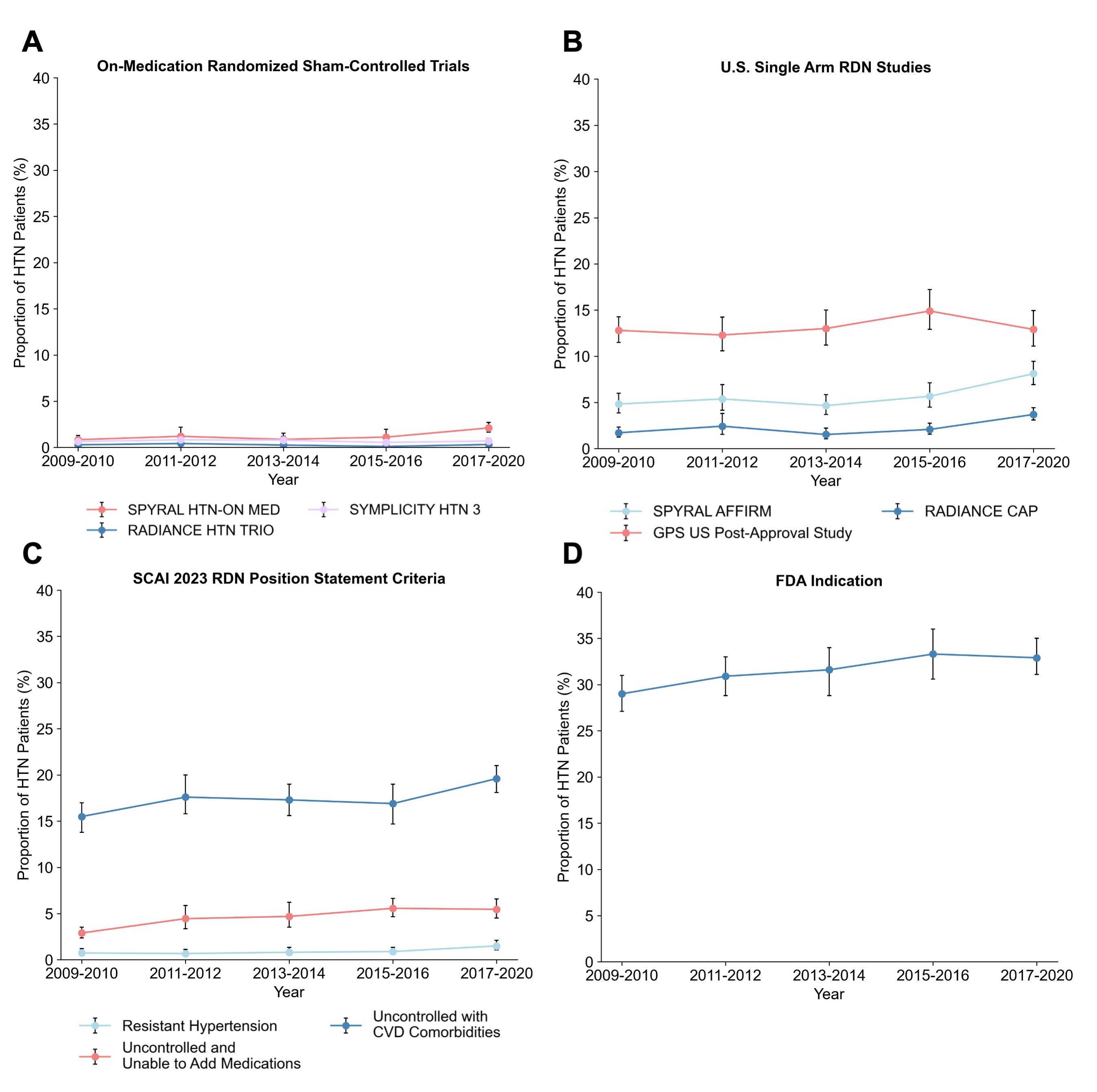
In total, we identified 16,677 patients with HTN in the US, representing a weighted total of 113,786,149 patients (Table). Using the FDA indication, 31.6% (95% CI, 30.7%-32.6%) of patients meet eligibility criteria for RDN, corresponding to 35,988,870 US adults. By the SCAI 2023 position statement selection criteria, 21.5% (95% CI, 20.7%-22.3%) of patients are eligible for consideration of RDN. Based on enrollment criteria from the RDN on-medication randomized trials, 2.05% (95% CI, 1.81%-2.33%) of US adults meet eligibility for consideration of RDN (Figure).
Conclusions
Our findings indicate that nearly one third of US adults with HTN are eligible for consideration of RDN based on the FDA indication; however, a smaller proportion of patients would be eligible based upon society recommendations and randomized trial inclusion criteria. Future studies are needed to further inform which patients will best benefit from this intervention.
Renal denervation (RDN) has been shown in randomized trials to improve blood pressure compared with a sham procedure. Currently, there are two FDA-approved RDN devices in the United States (US). While nearly half of the US population has hypertension (HTN), the number of patients who may benefit from RDN therapy remains uncertain. In this study, we used a nationally representative dataset to approximate the proportion of patients with HTN who may be eligible for consideration of RDN based on selective criteria.
Methods
All adult patients with HTN who participated in the National Health and Nutrition Examination Survey (NHANES) between the years 2009-2020 were identified. We characterized the proportion of these participants that met eligibility criteria based on 1) the FDA indication, 2) the SCAI 2023 RDN position statement, and 3) enrollment criteria from the RDN on-medication randomized trials. National estimates were obtained utilizing survey weighting from the NHANES multistage probability survey design.
Results
In total, we identified 16,677 patients with HTN in the US, representing a weighted total of 113,786,149 patients (Table). Using the FDA indication, 31.6% (95% CI, 30.7%-32.6%) of patients meet eligibility criteria for RDN, corresponding to 35,988,870 US adults. By the SCAI 2023 position statement selection criteria, 21.5% (95% CI, 20.7%-22.3%) of patients are eligible for consideration of RDN. Based on enrollment criteria from the RDN on-medication randomized trials, 2.05% (95% CI, 1.81%-2.33%) of US adults meet eligibility for consideration of RDN (Figure).
Conclusions
Our findings indicate that nearly one third of US adults with HTN are eligible for consideration of RDN based on the FDA indication; however, a smaller proportion of patients would be eligible based upon society recommendations and randomized trial inclusion criteria. Future studies are needed to further inform which patients will best benefit from this intervention.
More abstracts on this topic:
A cerebrovascular longitudinal atlas: different rates of morphological change in aneurysm patients associated with hypertension and diabetes
Chien Aichi, Salamon Noriko, Vinuela Fernando, Szeder Viktor, Colby Geoffrey, Jahan Reza, Boyle Noel, Villablanca Juan, Duckwiler Gary
Early renal denervation reduces ventricular arrhythmogenesis by improving Angiotensin II-disrupted expression of connexin 43 after acute myocardial infarctionDu Suiyong, Hu Wenfeng, Zhang Dongze