Final ID: MDP575
Temporal Trends and Predictors of Cardiac Biomarker Testing in the Emergency Department, 2014-2021
Abstract Body (Do not enter title and authors here): Background
Presenting symptoms risk stratify patients presenting to the Emergency Department (ED) to determine their likelihood of having a myocardial infarction (MI) and need for cardiac biomarker testing. Since the 2014 AHA/ACC non-ST segment elevation MI (NSTEMI) guideline, little is known regarding cardiac biomarker testing trends in the United States.
Methods
We analyzed data from the National Hospital Ambulatory Medical Care Survey for consecutive adult ED visits between 2014 and 2021 in the United States. The primary outcome was the proportion of ED visits where cardiac biomarker testing occurred. Subgroup analysis was performed for visits with chest pain, non-chest pain anginal equivalents, and non-anginal symptoms. Survey-weighted multivariable logistic regression was used to generate adjusted odds ratios (aOR) to assess predictors of cardiac biomarker utilization.
Results
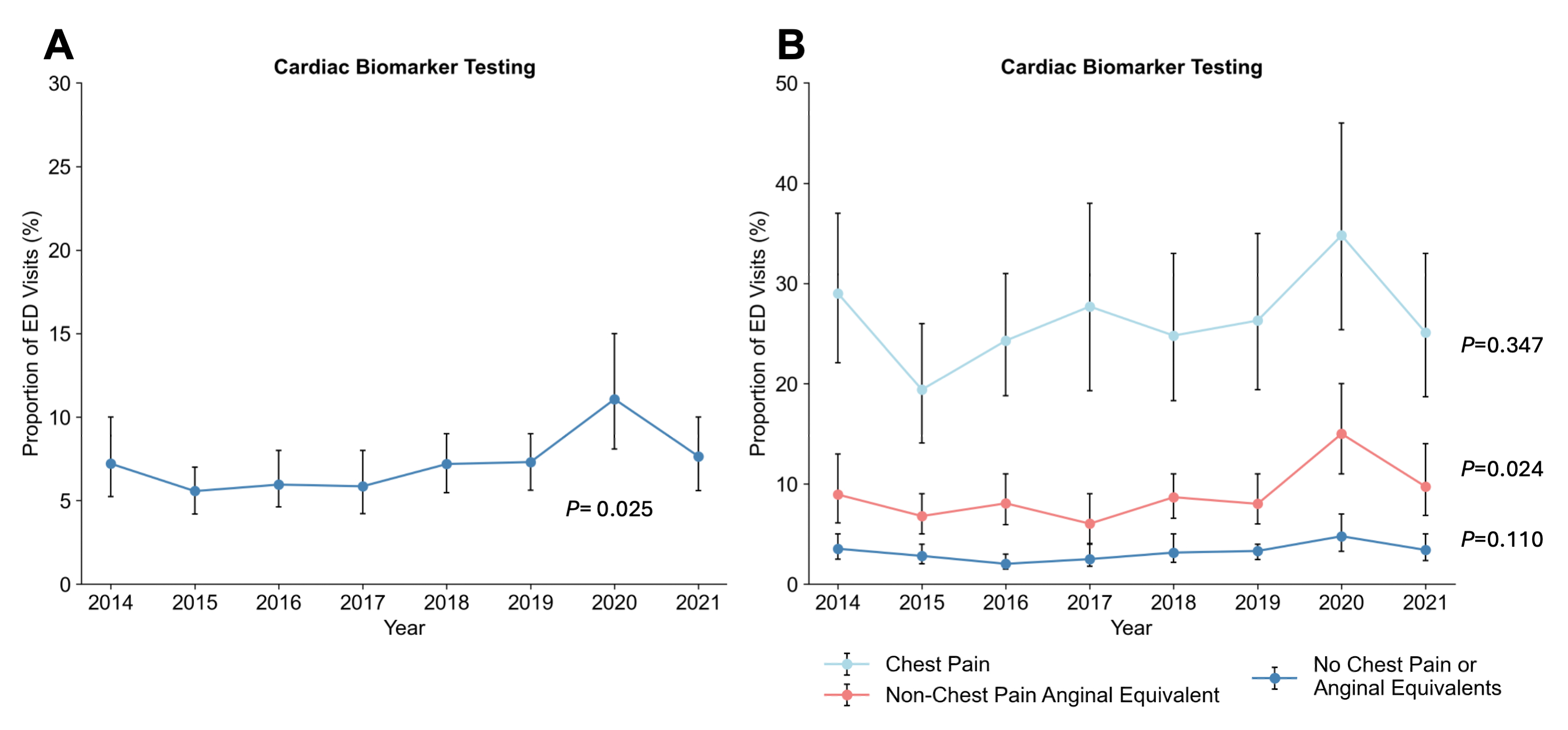
In total, 87,965 ED visits were sampled, representing a weighted total of 644,531,395 visits. Cardiac biomarker testing occurred in 7.21% of all ED visits (95% CI, 6.27%-8.29%) and increased over time (Figure, P=0.03 for trend). When stratified by symptoms, utilization of testing mainly increased among patients with non-chest pain anginal equivalents (Figure, P=0.02 for trend). Cardiac biomarker testing was most frequently used for patients with chest pain (34.9%) and non-chest pain anginal equivalents (39.3%). Nonetheless, 25.8% of patients who underwent testing had no chest pain or anginal equivalents. Cardiac biomarker testing was more frequent among patients with older age (aOR 1.03 per year, 95% CI 1.03-1.04), tachycardia (aOR 1.26, 95% CI 1.13-1.41), tachypnea (aOR 1.71, 95% CI 1.47-1.98), history of heart failure (aOR 1.33, 95% CI 1.15-1.54), prior venous thromboembolism (aOR 1.43, 95% CI 1.12-1.83), and those treated in a rural setting (aOR 1.63, 95% CI 1.14-2.32).
Conclusion
Cardiac biomarker testing in the ED has increased over time, mostly attributed to increased utilization among patients with a low pre-test probability of MI. Notably, 25% of individuals who underwent cardiac biomarker testing had no chest pain or anginal-equivalent symptoms. More judicious utilization of cardiac biomarker testing may be needed.
Presenting symptoms risk stratify patients presenting to the Emergency Department (ED) to determine their likelihood of having a myocardial infarction (MI) and need for cardiac biomarker testing. Since the 2014 AHA/ACC non-ST segment elevation MI (NSTEMI) guideline, little is known regarding cardiac biomarker testing trends in the United States.
Methods
We analyzed data from the National Hospital Ambulatory Medical Care Survey for consecutive adult ED visits between 2014 and 2021 in the United States. The primary outcome was the proportion of ED visits where cardiac biomarker testing occurred. Subgroup analysis was performed for visits with chest pain, non-chest pain anginal equivalents, and non-anginal symptoms. Survey-weighted multivariable logistic regression was used to generate adjusted odds ratios (aOR) to assess predictors of cardiac biomarker utilization.
Results
In total, 87,965 ED visits were sampled, representing a weighted total of 644,531,395 visits. Cardiac biomarker testing occurred in 7.21% of all ED visits (95% CI, 6.27%-8.29%) and increased over time (Figure, P=0.03 for trend). When stratified by symptoms, utilization of testing mainly increased among patients with non-chest pain anginal equivalents (Figure, P=0.02 for trend). Cardiac biomarker testing was most frequently used for patients with chest pain (34.9%) and non-chest pain anginal equivalents (39.3%). Nonetheless, 25.8% of patients who underwent testing had no chest pain or anginal equivalents. Cardiac biomarker testing was more frequent among patients with older age (aOR 1.03 per year, 95% CI 1.03-1.04), tachycardia (aOR 1.26, 95% CI 1.13-1.41), tachypnea (aOR 1.71, 95% CI 1.47-1.98), history of heart failure (aOR 1.33, 95% CI 1.15-1.54), prior venous thromboembolism (aOR 1.43, 95% CI 1.12-1.83), and those treated in a rural setting (aOR 1.63, 95% CI 1.14-2.32).
Conclusion
Cardiac biomarker testing in the ED has increased over time, mostly attributed to increased utilization among patients with a low pre-test probability of MI. Notably, 25% of individuals who underwent cardiac biomarker testing had no chest pain or anginal-equivalent symptoms. More judicious utilization of cardiac biomarker testing may be needed.
More abstracts on this topic:
A multi-proteomic Risk Score Predicts Adverse Cardiovascular Outcomes in Patients with Angina and Non-obstructive Coronary Artery Disease
Huang Jingwen, Lodhi Rafia, Lodhi Saleha, Eldaidamouni Ahmed, Hritani Wesam, Hasan Muhammet, Haroun Nisreen, Quyyumi Arshed, Mehta Puja, Leon Ana, Ko Yi-an, Yang Huiying, Medina-inojosa Jose, Ahmed Taha, Harris Kristen, Alkhoder Ayman, Al Kasem Mahmoud
A 50% or Greater Reduction in LDL-Cholesterol Is Associated with Improved Long-Term Outcomes and Lower Health Care Utilization After Myocardial Infarction - a SWEDEHEART studyReitan Christian, Watanabe Alexandre, Bash Lori, Galvain Thibaut, Arnet Urs, Jernberg Tomas