Final ID: We0022
Neutrophil Extracellular Traps Promote Aortic Aneurysm and Dissection by Modulating Vascular Smooth Muscle Cell Phenotype Change
Abstract Body: Background: Aortic dissection is a vascular disorder with high mortality, characterized by formation of a false lumen within the vessel wall. In aortic dissection, the microenvironment of the vessel wall undergoes dramatic changes including inflammatory cells infiltration and extracellular matrix remodeling. Recently, we have observed substantial formation of neutrophil extracellular traps (NETs) in aortic dissection, and inhibition of NETs could ameliorate aortic dissection. However, the mechanism by which NETs influence the progression of aortic dissection remains elusive.
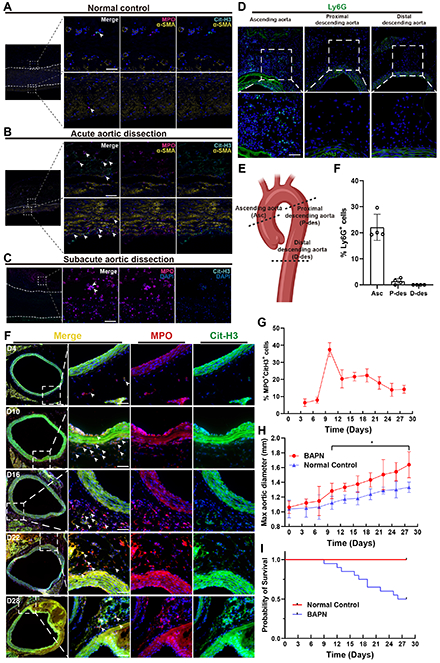
Methods: Immunofluorescence staining was performed on aorta from healthy controls and aortic dissection patients at different stages to assess NETs levels. A 0.5% β-Aminopropionitrile (BAPN) water-feeding model was used to induce aortic dissection in mice. Survival data was recorded. At various time points, NETs levels in mice aorta were assessed by immunofluorescence, and ascending aorta dilation was evaluated via echography. Neutrophils were purified from mouse bone marrow using density gradient centrifugation. NETs were induced by phorbol myristate acetate. Smooth muscle cells (SMCs) were isolated from mice throcic aorta. Phenotype change of SMCs was evaluated using quantitative real-time PCR, Western blotting, phalloidin staining, scratch assay, and gel contraction assay. The role of STAT3 signaling pathway in smooth muscle cell phenotypic change was investigated using small interfering RNA and overexpression plasmids.
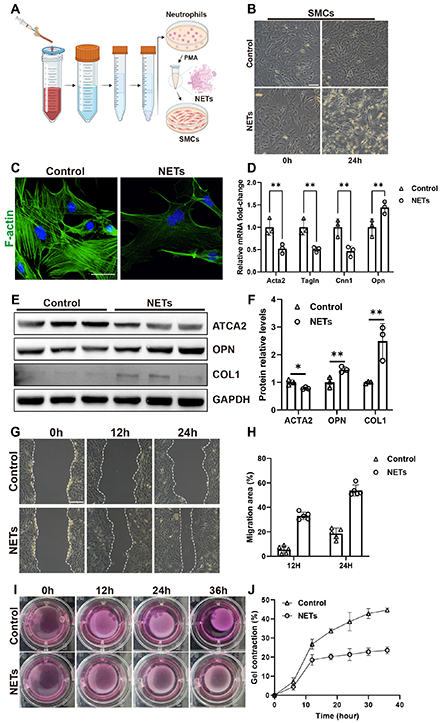
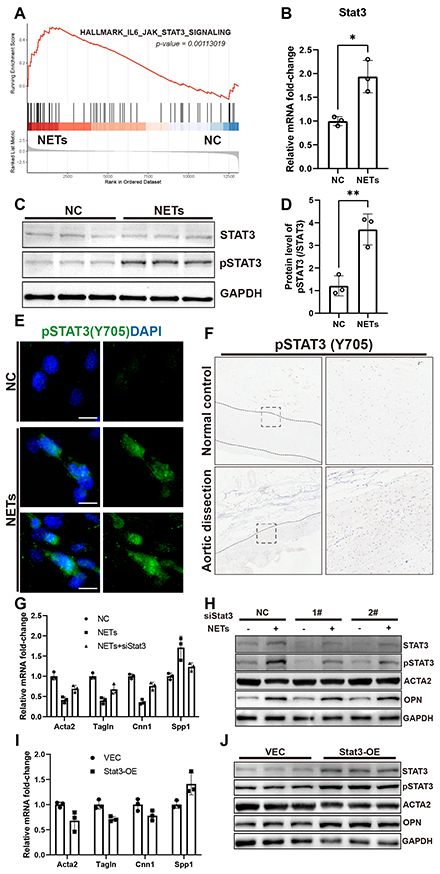
Results: The level of NETs in acute aortic dissection lesions was significantly higher compared to normal controls and subacute aortic dissection lesions. In BAPN water-feeding model, neutrophils infiltration was mainly observed in the ascending aorta, with a sharp increase of NETs on day 10, coinciding with the significant dilation of ascending aorta and the initiation of mice mortality. In vitro experiments showed that treating SMCs with NETs led to reduced contractility, with increased secretion and migration. Furthermore, NETs enhanced the activation of the STAT3 signaling pathway in SMCs, corroborated in human dissecting aorta. The critical role of STAT3 pathway activation in the phenotye change of SMCs was confirmed after siRNA-mediated knockdown and plasmid overexpression experiments.
Conclusions: NETs play a critical role in the early progression of aortic dissection by promoting phenotye change of aortic SMCs through activation of the STAT3 signaling pathway.
Methods: Immunofluorescence staining was performed on aorta from healthy controls and aortic dissection patients at different stages to assess NETs levels. A 0.5% β-Aminopropionitrile (BAPN) water-feeding model was used to induce aortic dissection in mice. Survival data was recorded. At various time points, NETs levels in mice aorta were assessed by immunofluorescence, and ascending aorta dilation was evaluated via echography. Neutrophils were purified from mouse bone marrow using density gradient centrifugation. NETs were induced by phorbol myristate acetate. Smooth muscle cells (SMCs) were isolated from mice throcic aorta. Phenotype change of SMCs was evaluated using quantitative real-time PCR, Western blotting, phalloidin staining, scratch assay, and gel contraction assay. The role of STAT3 signaling pathway in smooth muscle cell phenotypic change was investigated using small interfering RNA and overexpression plasmids.
Results: The level of NETs in acute aortic dissection lesions was significantly higher compared to normal controls and subacute aortic dissection lesions. In BAPN water-feeding model, neutrophils infiltration was mainly observed in the ascending aorta, with a sharp increase of NETs on day 10, coinciding with the significant dilation of ascending aorta and the initiation of mice mortality. In vitro experiments showed that treating SMCs with NETs led to reduced contractility, with increased secretion and migration. Furthermore, NETs enhanced the activation of the STAT3 signaling pathway in SMCs, corroborated in human dissecting aorta. The critical role of STAT3 pathway activation in the phenotye change of SMCs was confirmed after siRNA-mediated knockdown and plasmid overexpression experiments.
Conclusions: NETs play a critical role in the early progression of aortic dissection by promoting phenotye change of aortic SMCs through activation of the STAT3 signaling pathway.
More abstracts on this topic:
Can IVUS Predict Aortic Remodeling?
The Role of Flap Mobility in Type B Aortic Dissection With TEVAR
The Role of Flap Mobility in Type B Aortic Dissection With TEVAR
Kameda Yuika, Samejima Yusuke, Nemoto Naohiko, Anzai Hitoshi
Acute Electronic Cigarette Exposure Induces Heat Shock Response and Protein Aggregation in Vascular Smooth Muscle CellsDamiani Isabella, Huiting Wouter, Qin Guyu, Basu Sugandha, Easwaran Meena, Direnzo Elizabeth, Jarosz Daniel, Kim Juyong