Final ID: Tu0058a
OxLDL-CD36 Axis Induces Mitochondrial Translocation of Pyruvate Kinase M2 to Drive Reactive Oxygen Species in Macrophages
Abstract Body: Introduction: Atherosclerosis is characterized by the accumulation of pro-inflammatory macrophages within arterial walls, where they contribute to disease progression through the generation of reactive oxygen species (ROS) and chronic inflammation. Mitochondria are major sources of ROS in these macrophages, yet the molecular mechanisms underlying mitochondrial ROS (mtROS) production remain poorly understood. Our previous work demonstrated that the scavenger receptor CD36 enhances glycolysis and induces a threefold increase in mtROS in response to oxidized LDL (oxLDL). This led us to explore the link between glycolysis and mtROS induction in macrophages.
Hypothesis: Given that the glycolytic enzyme pyruvate kinase M2 (PKM2) has been implicated in oxidative stress and chronic inflammation, we hypothesize that the oxLDL/CD36 axis promotes mtROS production via PKM2 signaling.
Methods: Murine peritoneal macrophages and human monocyte-derived macrophages were analyzed using co-immunoprecipitation (co-IP), immunoblotting, immunostaining, proximity ligation assays, and flow cytometry-based mtROS measurements. Additionally, a mass spectrometry-based proteomics approach was employed to identify novel PKM2-interacting mitochondrial proteins.
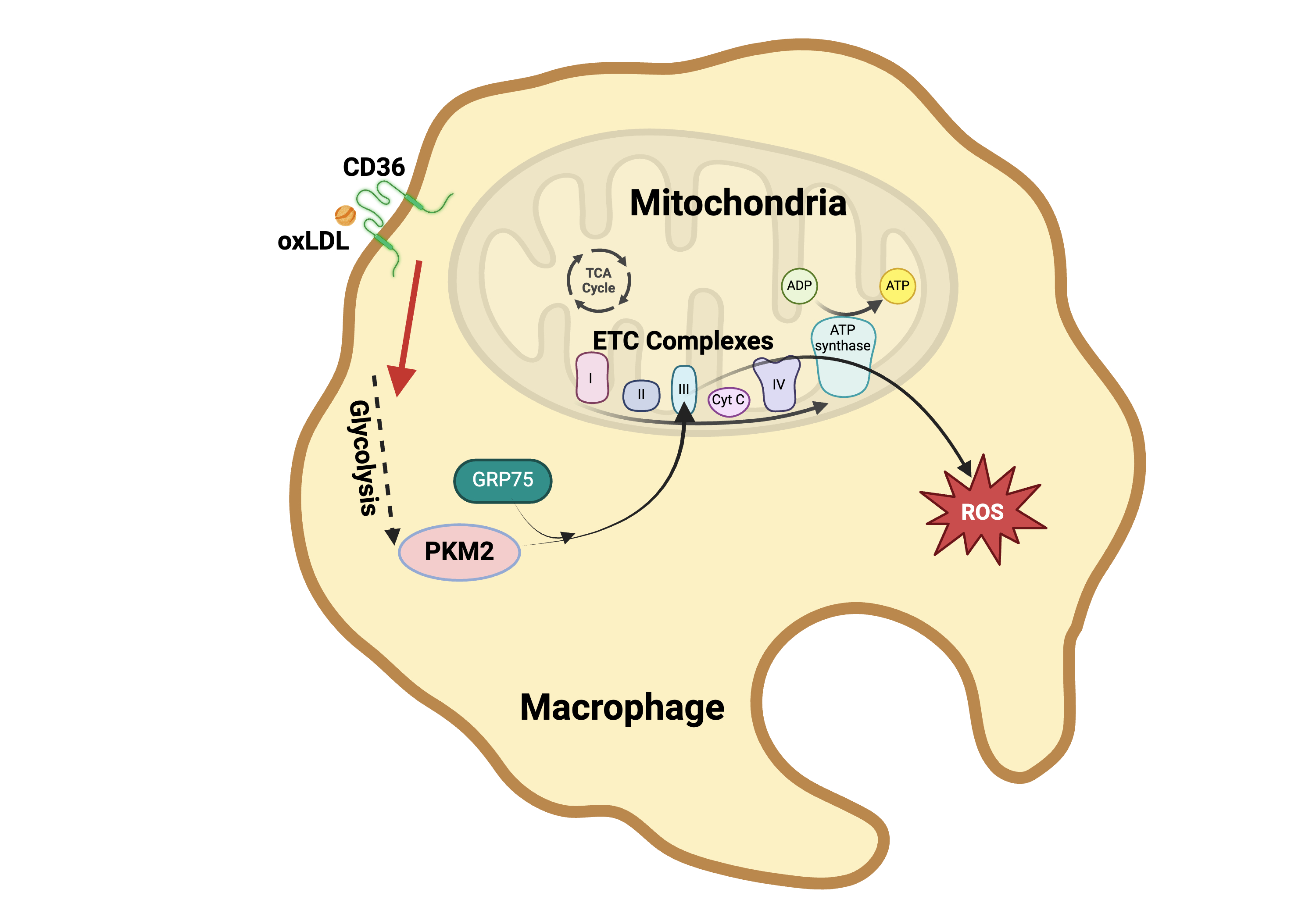
Results: Treatment of WT macrophages with oxLDL for 3 hours significantly increased mitochondrial PKM2 levels to 4.55 ± 0.90-fold compared to controls treated with native LDL (n=5). This increase was absent in Cd36-null macrophages. Mitochondrial localization of PKM2 was confirmed through co-immunostaining and proteinase K digestion assays, indicating that PKM2 localizes within the mitochondrial matrix, where it interacts with ETC complex III (n=4). PKM2 knockdown via siRNA or pharmacological inhibition completely abrogated oxLDL-induced mtROS production (n=5). Furthermore, co-IP, mass spectrometry, and in-situ proximity ligation assays revealed that oxLDL enhanced PKM2 binding to the chaperone protein GRP75. Silencing or inhibiting GRP75 disrupted PKM2 mitochondrial translocation and reduced mtROS levels in response to oxLDL (n=7).
Conclusions: Our findings unveil a novel oxLDL-CD36-PKM2 pathway that links CD36 signaling at the plasma membrane to mitochondrial dysfunction, driving mtROS production in macrophages (Figure 1). Targeting this pathway may offer a therapeutic strategy to mitigate macrophage-driven inflammation in atherosclerosis.
Hypothesis: Given that the glycolytic enzyme pyruvate kinase M2 (PKM2) has been implicated in oxidative stress and chronic inflammation, we hypothesize that the oxLDL/CD36 axis promotes mtROS production via PKM2 signaling.
Methods: Murine peritoneal macrophages and human monocyte-derived macrophages were analyzed using co-immunoprecipitation (co-IP), immunoblotting, immunostaining, proximity ligation assays, and flow cytometry-based mtROS measurements. Additionally, a mass spectrometry-based proteomics approach was employed to identify novel PKM2-interacting mitochondrial proteins.
Results: Treatment of WT macrophages with oxLDL for 3 hours significantly increased mitochondrial PKM2 levels to 4.55 ± 0.90-fold compared to controls treated with native LDL (n=5). This increase was absent in Cd36-null macrophages. Mitochondrial localization of PKM2 was confirmed through co-immunostaining and proteinase K digestion assays, indicating that PKM2 localizes within the mitochondrial matrix, where it interacts with ETC complex III (n=4). PKM2 knockdown via siRNA or pharmacological inhibition completely abrogated oxLDL-induced mtROS production (n=5). Furthermore, co-IP, mass spectrometry, and in-situ proximity ligation assays revealed that oxLDL enhanced PKM2 binding to the chaperone protein GRP75. Silencing or inhibiting GRP75 disrupted PKM2 mitochondrial translocation and reduced mtROS levels in response to oxLDL (n=7).
Conclusions: Our findings unveil a novel oxLDL-CD36-PKM2 pathway that links CD36 signaling at the plasma membrane to mitochondrial dysfunction, driving mtROS production in macrophages (Figure 1). Targeting this pathway may offer a therapeutic strategy to mitigate macrophage-driven inflammation in atherosclerosis.
More abstracts on this topic:
Endotoxemia induces mitochondrial DNA (mtDNA) damage in mouse myocardium and cardiomyocytes
Dubey Praveen, Singh Sarojini, Meeler Avery, Krishnamurthy Prasanna
Acute loss of PGC1α in adult cardiomyocytes reduces ischemia-reperfusion injuryHe Lihao, Young Martin E, Rowe Glenn, Prabhu Sumanth, Sethu Palaniappan, Xie Min, Chen Yunxi, Chu Yuxin, Hua Yutao, Cai Junyan, He Jin, Benavides Gloria, Darley-usmar Victor, Ballinger Scott