Final ID: Or109
Systemic Nicotinamide Mononucleotide Administration for Post-cardiac Arrest Brain Injury
Abstract Body: Background: Nicotinamide mononucleotide (NMN), a precursor of nicotinamide adenine dinucleotide (NAD+), has been shown to increase NAD+ levels, reduce inflammation, and improve short-term survival in a rodent model of hemorrhagic shock. NAD+ levels decrease after cardiac arrest (CA), but the effect of NMN on outcomes after CA remains undefined.
Hypothesis: NMN administration increases NAD+ content in the brain, reduces systemic inflammation, and improves outcomes after CA.
Aims: This study aimed to investigate the effects of systemic NMN administration on neurological function, survival, and systemic inflammation after CA.
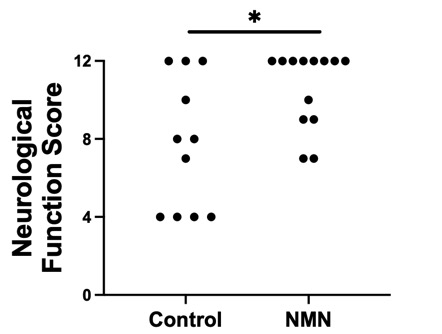
Methods: In a murine model of CA, asystole was induced using potassium chloride. After 10 minutes of CA, mice were resuscitated with continuous epinephrine injections. Mice were randomly assigned to the NMN group (60 mg/kg body weight i.p.) or the control group (normal saline i.p.) 1.5 minutes after the return of spontaneous circulation (ROSC). The same treatment was repeated at 24 and 48 hours after CA. Neurological function score (on a scale from 0 to 12) at 48 hours post-CA and 7-day survival were compared between the NMN and control groups. Brain NAD+ levels were measured 30 minutes post-ROSC. Plasma cytokine levels (IL-6 and TNF-α) were measured 2 hours post-ROSC.
Results: Brain NAD+ levels significantly increased 30 minutes post-ROSC in the NMN group compared to the control group (186 ± 15 pg/mg tissue and 131 ± 14 pg/mg tissue, respectively; P=0.02). NMN significantly improved neurological function score at 48 hours post-CA (NMN group median 12 [9–12] vs. control group 8 [4–11]; P=0.03). Moreover, NMN improved survival rate up to 7 days post-CA (NMN group 61.1% [11/18] vs. control group 22.2% [4/18]; P=0.03). Mean arterial pressure tended to be higher in the NMN group, although the difference was not significant (NMN group 113.8 ± 2.1 mmHg vs. control group 107.8 ± 2.9 mmHg; P=0.08). NMN showed a trend toward decreased IL-6 (NMN group 52.7 ± 14.3 pg/ml vs. control group 114.6 ± 33.3 pg/ml; P=0.15) and TNF-α (NMN group 6.9 ± 1.2 pg/ml vs. control group 11.7 ± 2.3 pg/ml; P=0.12).
Conclusions: Systemic administration of NMN post-CA increased brain NAD+ levels and improved neurological function and survival. NMN also showed a trend toward reduced systemic inflammation. NMN is a promising approach to improve outcomes after CA.
Hypothesis: NMN administration increases NAD+ content in the brain, reduces systemic inflammation, and improves outcomes after CA.
Aims: This study aimed to investigate the effects of systemic NMN administration on neurological function, survival, and systemic inflammation after CA.
Methods: In a murine model of CA, asystole was induced using potassium chloride. After 10 minutes of CA, mice were resuscitated with continuous epinephrine injections. Mice were randomly assigned to the NMN group (60 mg/kg body weight i.p.) or the control group (normal saline i.p.) 1.5 minutes after the return of spontaneous circulation (ROSC). The same treatment was repeated at 24 and 48 hours after CA. Neurological function score (on a scale from 0 to 12) at 48 hours post-CA and 7-day survival were compared between the NMN and control groups. Brain NAD+ levels were measured 30 minutes post-ROSC. Plasma cytokine levels (IL-6 and TNF-α) were measured 2 hours post-ROSC.
Results: Brain NAD+ levels significantly increased 30 minutes post-ROSC in the NMN group compared to the control group (186 ± 15 pg/mg tissue and 131 ± 14 pg/mg tissue, respectively; P=0.02). NMN significantly improved neurological function score at 48 hours post-CA (NMN group median 12 [9–12] vs. control group 8 [4–11]; P=0.03). Moreover, NMN improved survival rate up to 7 days post-CA (NMN group 61.1% [11/18] vs. control group 22.2% [4/18]; P=0.03). Mean arterial pressure tended to be higher in the NMN group, although the difference was not significant (NMN group 113.8 ± 2.1 mmHg vs. control group 107.8 ± 2.9 mmHg; P=0.08). NMN showed a trend toward decreased IL-6 (NMN group 52.7 ± 14.3 pg/ml vs. control group 114.6 ± 33.3 pg/ml; P=0.15) and TNF-α (NMN group 6.9 ± 1.2 pg/ml vs. control group 11.7 ± 2.3 pg/ml; P=0.12).
Conclusions: Systemic administration of NMN post-CA increased brain NAD+ levels and improved neurological function and survival. NMN also showed a trend toward reduced systemic inflammation. NMN is a promising approach to improve outcomes after CA.
More abstracts on this topic:
Attenuating Post-stroke Ischemia Reperfusion Injury: Establishing the Efficacy of Disodium Malonate in a Clinically Relevant Sheep Model
Sorby-adams Annabel, Murphy Mike, Sharkey Jessica, Prag Hiran, Turner Renee, Skein Keziah, Guglietti Bianca, Pullan Caitlin, Williams Georgia, Krieg Thomas
A Porcine Model of Cardiac Arrest Without Pre-Arrest Fluid Loading, Sternal Molding, or EpinephrineParadis Aidan, Paradis Norman, Gaddy David, Moodie Karen, Mader Timothy, Dufresne Alexandre, Couturier Christine, Dufresne Simon, Davis Daniel, Sims Christopher