Final ID: OGCTP34
Cerebral Autoregulation-guided Individualized Blood Pressure Management After Successful Recanalization For Patients With Large Vessel Occlusion Stroke: the RESCUE-CA Phase II Proof-of-concept Study
In patients with large vessel occlusion stroke, fixed-target blood pressure (BP) reduction post-revascularization has shown limited benefits and may be even harmful. Observational studies have highlighted autoregulation-guided, individualized BP management as a promising alternative. This study seeks to determine if an autoregulation-based BP target is both safe and more effective than a fixed target for patients with ischemic stroke following endovascular thrombectomy (EVT).
Methods
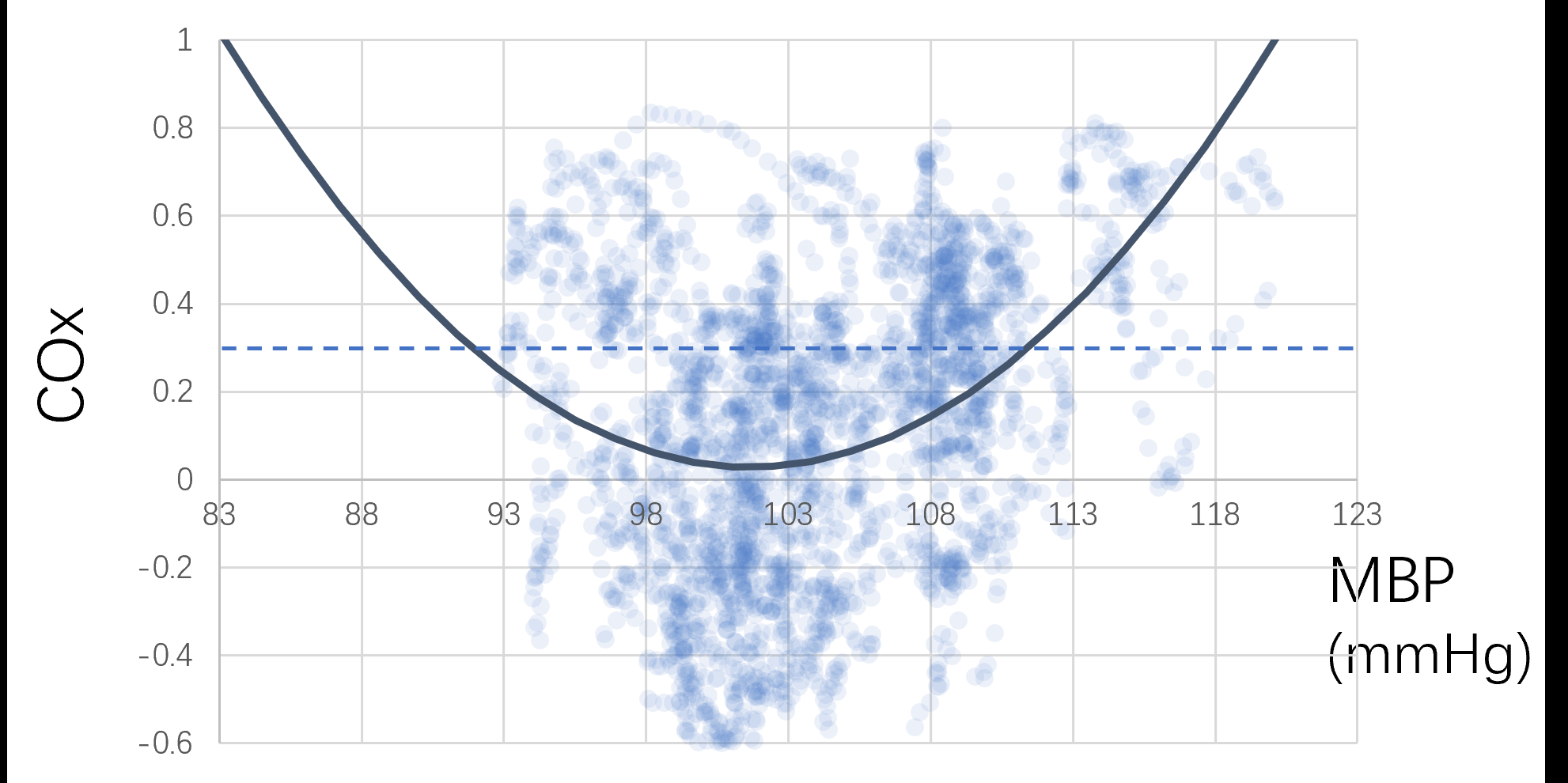
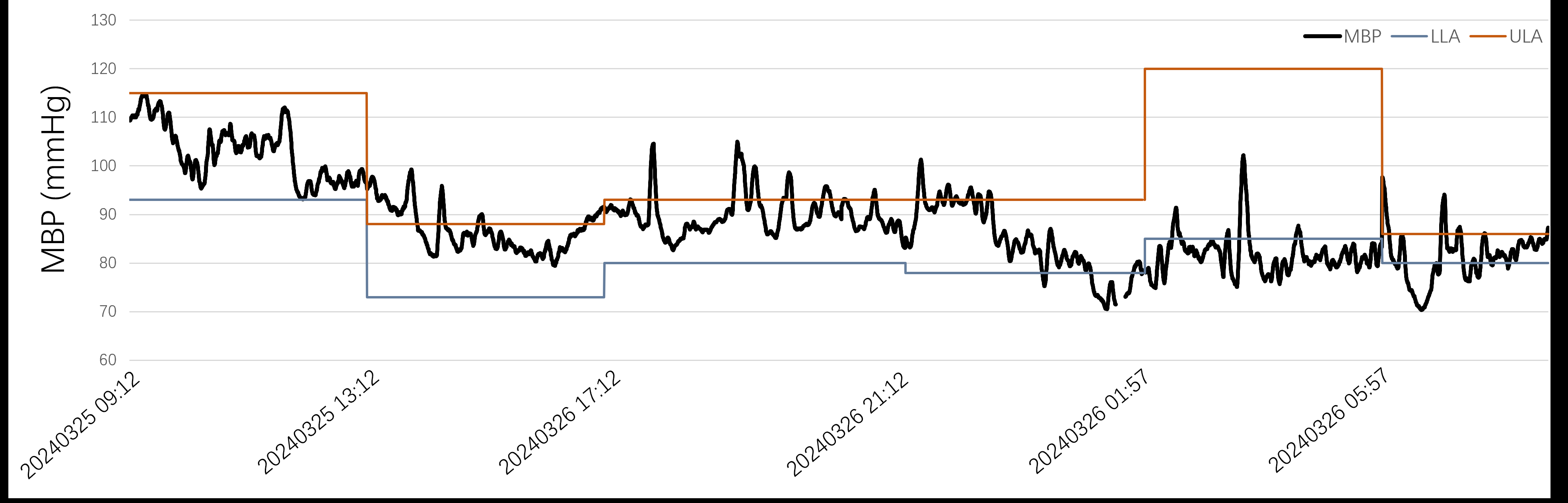
This prospective, single-center, open-label, randomized controlled proof-of-concept trial aims to evaluate the efficacy of a tailored BP strategy based on cerebral autoregulation. Patients with ICA or MCA-M1 occlusion who achieve successful recanalization (mTICI 2b-3) via EVT are randomized within 8 hours post-procedure. The control group follows current guideline-based BP targets (<180/105 mmHg), while the autoregulation-guided BP group has personalized BP targets set by cerebrovascular autoregulation limits identified through COx (the Pearson correlation between arterial pressure and brain tissue oxygenation index). BP is monitored and adjusted every four hours for 48 hours post-EVT. The hypotheses of RESCUE-CA are, compared to controls, the autoregulation-guided group will exhibit (1) reduced risk of significant infarct growth (volume growth > 11.6 mL) at 7 days, (2) lower incidence of symptomatic intracerebral hemorrhage within 72 hours, (3) reduced early neurological deterioration (≥2-point NIHSS scores increase) at 7 days, and (4) improved outcomes (mRS scores 3-6) at 90 days.
Ethics and Dissemination
This study has been approved by the Ethics Committee of Beijing Tiantan Hospital. Findings will be disseminated at international conferences and published in peer-reviewed journals.
Trial registration: NCT05670028
More abstracts on this topic:
Maria Shannon, Mojares Joseph, Zrelak Patricia
A distinct clot transcriptomic signature is associated with atrial fibrillation-derived ischemic stroke in the INSIGHT RegistrySeah Carina, Rivet Dennis, Fraser Justin, Kellner Christopher, Devarajan Alex, Vicari James, Dabney Alan, Baltan Selva, Sohrabji Farida, Pennypacker Keith, Nanda Ashish, Woodward Britton
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.