Final ID: OGCTP18
Randomized Trial of Milvexian versus Placebo in Acute Ischemic Stroke and Transient Ischemic Attack: Rationale and Methods for the LIBREXIA-Stroke Trial
Objective: The ongoing phase 3 LIBREXIA-Stroke Trial (ClinicalTrials.gov: NCT05702034; sponsored by Bristol Myers Squibb and Janssen Research & Development, LLC) is addressing whether milvexian is superior to placebo in reducing the risk of subsequent ischemic stroke among patients presenting acutely with ischemic stroke or high-risk TIA.
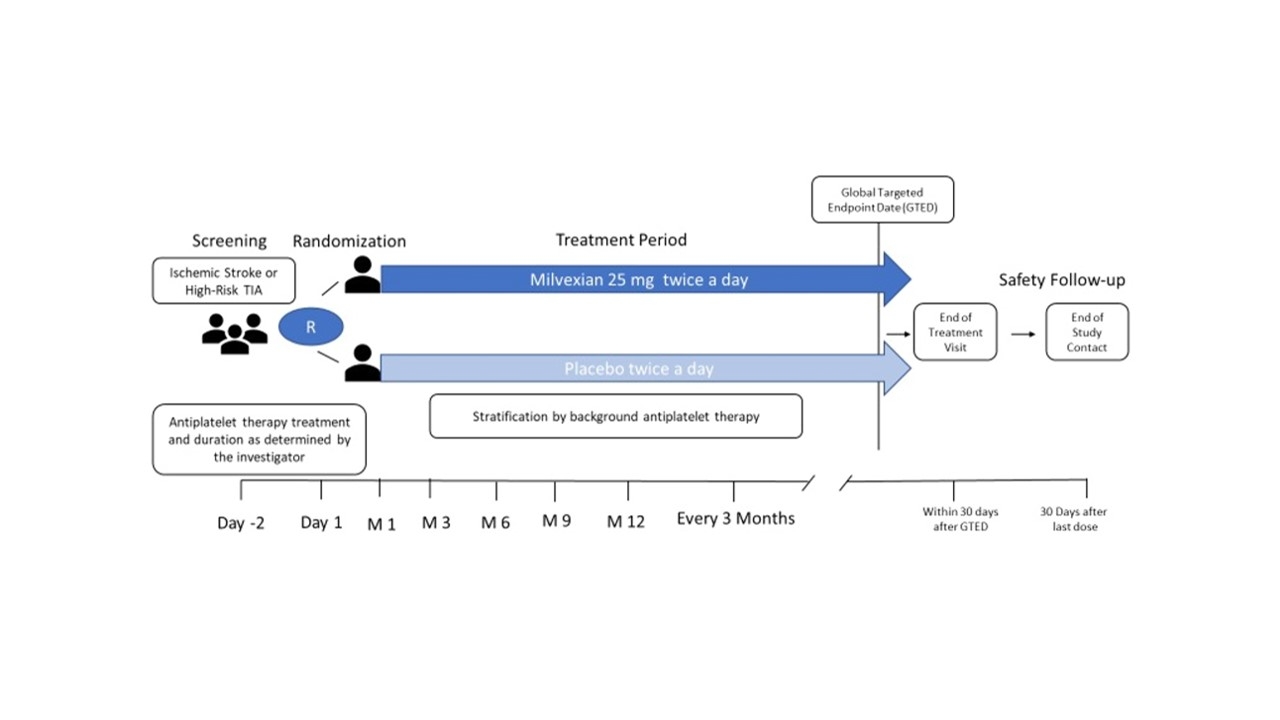
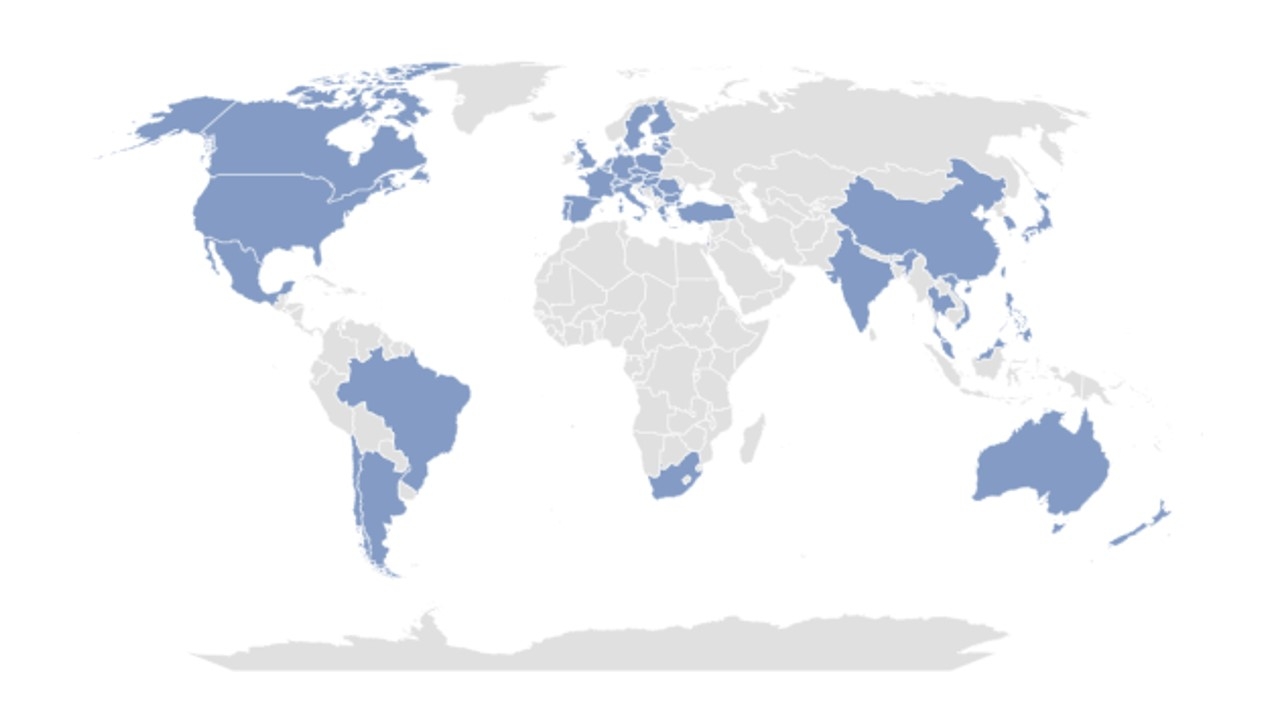
Design: LIBREXIA-Stroke is an international, double-blind, placebo-controlled trial in patients with acute non-cardioembolic ischemic stroke (NIHSS ≤7) or high-risk TIA (ABCD2 score ≥6) who are randomized within 48 hours of symptom onset to milvexian (25 mg twice daily) or matching placebo, with all patients receiving background antiplatelet therapy (Figure 1). Patients undergoing thrombolysis or thrombectomy are eligible if enrolled more than 24 hours after the procedure. The primary efficacy outcome is time to first occurrence of ischemic stroke, and the principal safety outcome is BARC 3c or 5 hemorrhage (intracranial, symptomatic intraocular, or fatal hemorrhage). Patients with an elevated risk of hemorrhage at screening are excluded. For this outcome event-driven trial, it was estimated that approximately 15,000 subjects will be randomized in 47 countries (Figure 2) and followed until 909 primary efficacy outcome events have occurred to achieve 90% power to detect a 20% hazard reduction with milvexian.
Conclusions: The LIBREXIA-Stroke trial will assess the efficacy and safety of milvexian. This new anticoagulant may have a favorable risk-benefit profile in a large international population of patients presenting acutely with ischemic stroke or high-risk TIA.
- Johnston, S. Claiborne ( Department of Neurology, University of California , San Francisco , California , United States )
- Lu, Wentao ( Janssen Research & Development, LLC, a Johnson & Johnson Company , Raritan , New Jersey , United States )
- Li, Danshi ( Bristol Myers Squibb , Princeton , New Jersey , United States )
- Plotnikov, Alexei ( Janssen Research & Development, LLC, a Johnson & Johnson Company , Raritan , New Jersey , United States )
- Gibson, Charles ( Harvard Medical School , Boston , Massachusetts , United States )
- Lam, Carolyn ( National Heart Centre Singapore & Duke-National University of Singapore , Singapore , Singapore )
- Mahaffey, Kenneth ( Stanford University School of Medicine , Stanford , California , United States )
- Mehran, Roxana ( Mount Sinai School of Medicine , New York , New York , United States )
- Steg, Philippe ( Hôpitaux de Paris , Paris , France )
- Weitz, Jeffrey ( McMaster University , Hamilton , Ontario , Canada )
- Harrington, Robert ( Weill Cornell Medicine , New York , New York , United States )
- De Silva, Deidre ( National Neuroscience Institute, Singapore General Hospital , Bukit Merah , Singapore )
- Hankey, Graeme ( Centre for Neuromuscular and Neurological Disorders, Medical School, The University of Western Australia , Perth , Western Australia , Australia )
- Furie, Karen ( Rhode Island Hospital, Warren Alpert Medical School of Brown University , Providence , Rhode Island , United States )
- James, Stefan ( Uppsala University , Uppsala , Sweden )
- Martins, Sheila ( Hospital de Clínicas de Porto Alegre , Porto Alegre , Brazil )
- Molina, Carlos ( Vall d'Hebron Research Institute (VHIR) , Barcelona , Spain )
- Wang, Yongjun ( Beijing Tian Tan Hospital , Beijing , China )
- Yavin, Yshai ( Janssen Research & Development, LLC, a Johnson & Johnson Company , Raritan , New Jersey , United States )
- Albanese, John ( Janssen Research & Development, LLC, a Johnson & Johnson Company , Raritan , New Jersey , United States )
Meeting Info:
Session Info:
More abstracts on this topic:
Bourassa Sharon, Worthington Michelle
Antithrombotic Strategies for Stroke Prevention in Elderly Patients with Atrial Fibrillation: A Meta-Analysis of Contemporary EvidenceFarooq Talha, Pandit Maleeha, Ali Mohammad Eisa, Ahsan Muhammad, Khan Abdul Moiz, Qasim Muhammad, Qayyum Mahhum, Akram Anusha, Kamel Mohammed, Naseem Ali
More abstracts from these authors:
Lam Carolyn
Impact of Hyper-Polypharmacy on Cardiovascular Outcomes in Patients With Acute Coronary Syndrome: Analysis Form the ATLAS ACS 2-TIMI 51 TrialChi Gerald, Plotnikov Alexei, Gibson Charles
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.