Final ID: OGCTP24
NIH StrokeNet
Abstract Body: BACKGROUND AND AIMS: NINDS established NIH StrokeNet in 2013 to harness the leadership and experience of the stroke field to efficiently create and complete high-quality, multi-site trials and related biomarker validation and ancillary studies spanning prevention, treatment, and recovery. The network continues to grow its trial portfolio and increase the activity of its collaborative groups.
METHODS: The infrastructure currently consists of the National Coordinating Center with pharmacy and imaging management, and single IRBs and contracting functions; the National Data Management Center; 27 Regional Coordinating Centers with over 550 affiliated sites; and the NIH/NINDS that provides administrative oversight and scientific input. Working Group Committees consist of Acute, Prevention, and Recovery/Rehabilitation. Cores consist of Fellow Education/Training, Clinical Research Professional Education/Training, and Diversity, Equity, and Inclusion. Advisory Committees consist of Patient Representation and Advocacy, Pediatrics, Preclinical Science, and TeleStroke. The network also stewards the Global Alliance of Independent Networks focused on Stroke trials (GAINS).
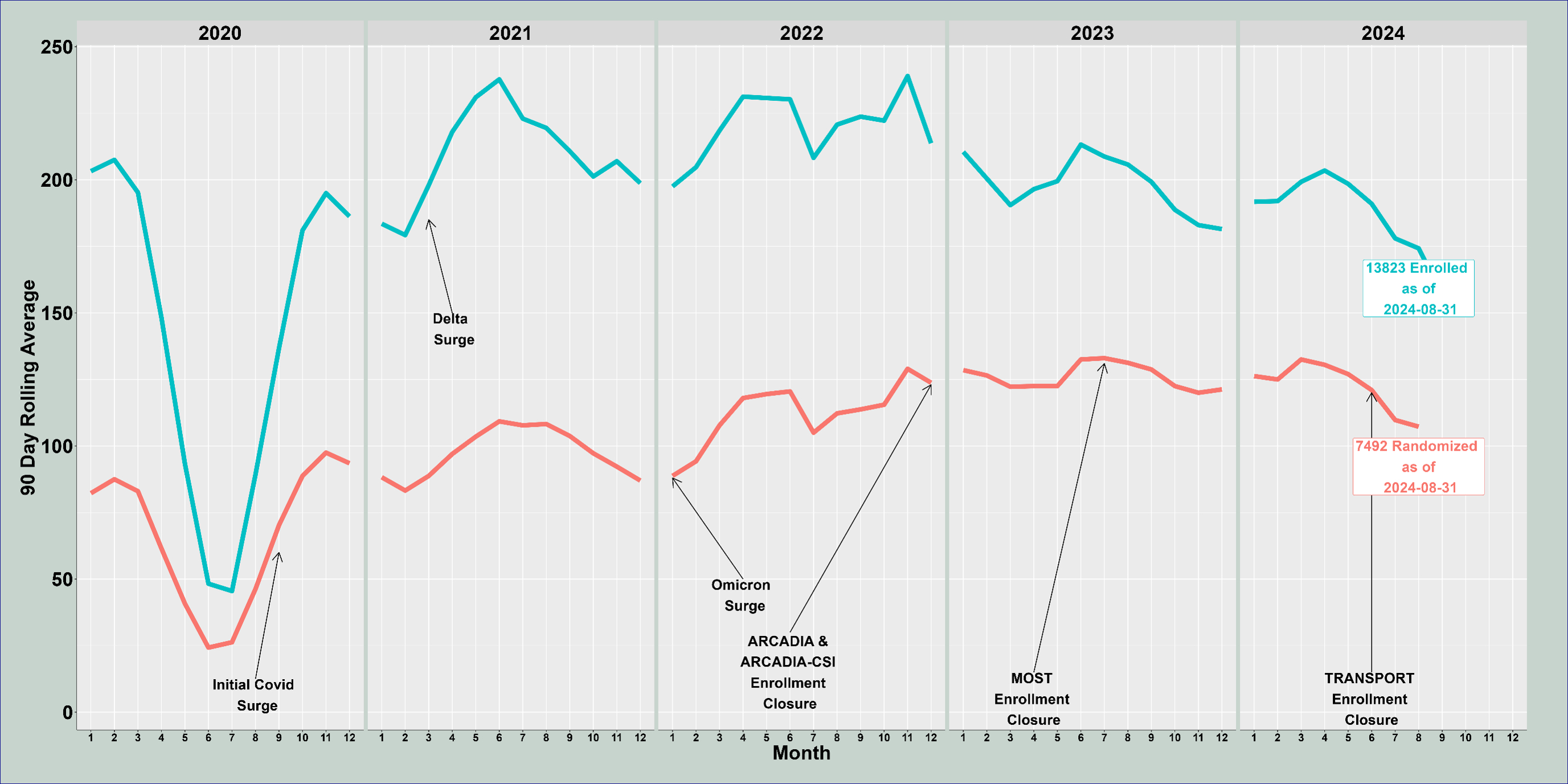
RESULTS: As of October 30, 2024, 13,589 participants were enrolled, and 6,762 were randomized, in StrokeNet studies from 273 US and 43 non-US sites. Thirteen studies are in start-up/recruitment in Prevention (CREST-2, CREST-H, Sleep-SMART, SATURN, SATURN-MRI, ASPIRE, CAPTIVA and FOCAS), Acute (FASTEST, SISTER and STEP), and Recovery/Rehab (VERIFY and TELEREHAB-2) domains. Three studies are in follow-up/closeout: TRANSPORT-2, I-ACQUIRE and ARCADIA-CSI. Eight studies are completed: MISTIE-3, DEFUSE 3, I-DEF, TELEREHAB, ACTIV-4C, ACTIV 4A, ARCADIA and MOST. To date, NIH StrokeNet has partnered with seven countries outside the US and seven companies. Most StrokeNet studies have had adaptive statistical design features, and a platform trial is ongoing.
CONCLUSIONS: NIH StrokeNet has demonstrated the ability to design, implement and complete innovative and impactful stroke studies.
METHODS: The infrastructure currently consists of the National Coordinating Center with pharmacy and imaging management, and single IRBs and contracting functions; the National Data Management Center; 27 Regional Coordinating Centers with over 550 affiliated sites; and the NIH/NINDS that provides administrative oversight and scientific input. Working Group Committees consist of Acute, Prevention, and Recovery/Rehabilitation. Cores consist of Fellow Education/Training, Clinical Research Professional Education/Training, and Diversity, Equity, and Inclusion. Advisory Committees consist of Patient Representation and Advocacy, Pediatrics, Preclinical Science, and TeleStroke. The network also stewards the Global Alliance of Independent Networks focused on Stroke trials (GAINS).
RESULTS: As of October 30, 2024, 13,589 participants were enrolled, and 6,762 were randomized, in StrokeNet studies from 273 US and 43 non-US sites. Thirteen studies are in start-up/recruitment in Prevention (CREST-2, CREST-H, Sleep-SMART, SATURN, SATURN-MRI, ASPIRE, CAPTIVA and FOCAS), Acute (FASTEST, SISTER and STEP), and Recovery/Rehab (VERIFY and TELEREHAB-2) domains. Three studies are in follow-up/closeout: TRANSPORT-2, I-ACQUIRE and ARCADIA-CSI. Eight studies are completed: MISTIE-3, DEFUSE 3, I-DEF, TELEREHAB, ACTIV-4C, ACTIV 4A, ARCADIA and MOST. To date, NIH StrokeNet has partnered with seven countries outside the US and seven companies. Most StrokeNet studies have had adaptive statistical design features, and a platform trial is ongoing.
CONCLUSIONS: NIH StrokeNet has demonstrated the ability to design, implement and complete innovative and impactful stroke studies.
More abstracts on this topic:
A Fat Chance: Paradoxical Embolic Stroke from Lipomatous Hypertrophy of the Interatrial Septum
Kalathoor Abraham
A Delayed Diagnosis of Anti-HMG-CoA Reductase Immune-Mediated Necrotizing MyopathyJadhav Reshma, Shekar Arush, Westenhaver Zack, Skandhan Amith
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)