Final ID: WP117
AI-driven Electromagnetic Field Therapy to Reduce Global Disability in Patients with Subacute Ischemic Stroke: Trajectory of Potential Benefit in the EMAGINE 1 Trial
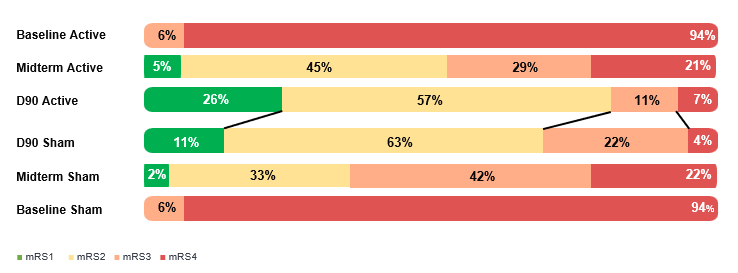
Methods: We conducted a multicenter, double-blind, randomized, sham-controlled, trial enrolling participants 4-21 days post-stroke with baseline modified Rankin Scale 3-4 and Fugl-Meyer Assessment Upper-Extremity 10-45. Participants were allocated to active or sham treatment, of stimulation paired with an evidence-based, functional, repetitive, home-based physical exercises regimen for 45 one-hour sessions, five times per week within the first 90 days from stroke. Global disability mRS was assessed at baseline, day 45, and day 90.
Results: Participant age was 59.0 (+12.5), 33% were female, and study treatment was initiated at day 14 (IQR 12-19) post-stroke. The evolution of mRS distribution in the active and sham stimulation groups is shown in the Figure. At day 45, there was evidence of potential treatment benefit for functional independence (mRS 0-2, active vs sham 50% vs 35%, adjusted p=0.05) though not in freedom-from-disability (mRS 0-1, 5% vs 2%). By day 90, after further recovery in both treatment groups, there was evidence of potential treatment benefit for both freedom-from-disability (mRS 0-1, 26% vs 11%, adjusted p=0.03) and functional independence (mRS 0-2, 83% vs 74%, p=0.13). Shift on the mRS trichotomized at 0-1/2/3-6 similarly showed signals of benefit at day 45 (adjusted p=0.07) in addition to day 90 (adjusted p=0.02).
Conclusion: ENTF therapy showed safety and preliminary efficacy in reducing global disability among subacute ischaemic stroke patients with severe baseline disability, with potential benefit accruing at mRS 0-2 by day 45 and at both mRS 0-1 and 0-2 by day 90. These results require confirmation in an adequately powered prospective trial.
More abstracts on this topic:
Ferrone Nicholas, Sanmartin Maria, O'hara Joseph, Jimenez Jean, Ferrone Sophia, Wang Jason, Katz Jeffrey, Sanelli Pina
A Randomized Comparison of Online Motivational Themes in Cardiovascular Clinical Trial RecruitmentHussain Zaib, Harry Tamunotonye, Michos Erin, Milller Hailey, Juraschek Stephen, Turkson-ocran Ruth-alma, Lahey Timothy, Feng Yuanyuan, Plante Timothy
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.