Final ID: WP333
Population-based Study of Stroke Recurrence in Overweight or Obese Patients: Considerations for Future Prevention Trials
Recurrent stroke accounts for approximately 1/4 of all strokes and is associated with high morbidity and mortality. Glucagon-like peptide-1 receptor agonists (GLP-1), while originally developed to treat diabetes, have demonstrated efficacy in preventing cardiovascular events in overweight or obese (BMI>27) patients without diabetes. It is unknown whether these agents could also be useful for preventing recurrence in non-diabetic overweight or obese stroke patients. To guide potential trial planning, we sought to characterize the prevalence of overweight or obesity without diabetes among stroke patients and assess the recurrence rate in these patients at 3 years.
METHODS:
Using the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS) database from (2015), we identified adult patients with a diagnosis of acute ischemic stroke (AIS) or transient ischemic attack (TIA) and a BMI >27. Patients were separated into two groups based on whether they had a diagnosis of diabetes (either a prior history or a new diagnosis). Demographic information, premorbid mRS, stroke subtype, vascular risk factors and rate of stroke recurrence at 3 years were analyzed. We calculated Kaplan Meier estimates of 3-year recurrence in stroke patients with BMI>27 with and without diabetes. We used a log-rank test to test if there was a difference in the rate of recurrence between the two groups.
Results:
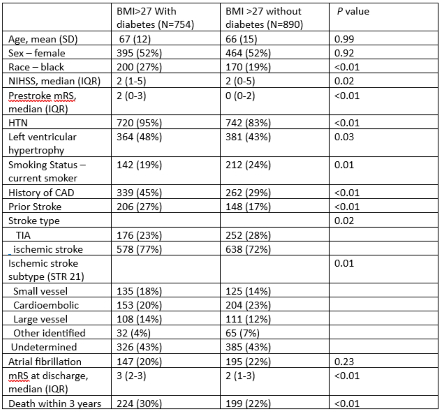
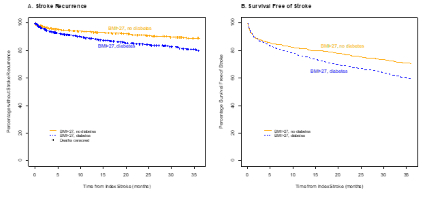
In 2015, of 3086 patients with an AIS or TIA, 3057 had BMI reported and 1644 (54%) with a BMI>27. 859 (52%) of these patients were female, 370 (23%) were Black, and 754 (46%) of these patients had a prior or new diagnosis of diabetes. Overweight or obese patients without diabetes differed from diabetics in race, baseline stroke severity, baseline disability, frequency of HTN and LVH, CAD, smoking and prior stroke. Unadjusted risk of recurrence in non-diabetic overweight or obese patients was 11% (95% CI: 8%, 15%) at 3 years, which was lower than the rate in diabetic overweight or obese of 20% (95% CI: 16%, 26%) (log-rank test p-value <0.01). Unadjusted survival free of stroke in non-diabetic overweight or obese patients was 71% (95% CI: 66%, 75%) at 3 years, which was higher than in diabetic overweight or obese patients of 59% (95% CI: 53%, 65%).
Conclusion:
Of all ischemic stroke and TIA patients, 29% have a BMI>27 without diabetes; these patients have a 3-year stroke recurrence rate of 11% and survival free of stroke of 71%. Clinical trials targeting this group are needed.
- Yahnke, Ian ( University of Cincinnati , Cincinnati , Ohio , United States )
- Demel, Stacie ( University of Cincinnati , Cincinnati , Ohio , United States )
- Mistry, Eva ( University of Cincinnati , Cincinnati , Ohio , United States )
- Coleman, Elisheva ( University of Chicago , Cincinnati , Ohio , United States )
- Jasne, Adam ( Yale Stroke Center , New Haven , Connecticut , United States )
- Slavin, Sabreena ( University of Kansas Medical Center , Kansas City , Kansas , United States )
- Walsh, Kyle ( University of Cincinnati , Cincinnati , Ohio , United States )
- Star, Michael ( Soroka Medical Center , Beersheva , Israel )
- Stanton, Robert ( University of Cincinnati , Cincinnati , Ohio , United States )
- Ljungberg, Lovisa ( University of Cincinnati , Cincinnati , Ohio , United States )
- Kamel, Hooman ( Weill Cornell Medicine , New York , New York , United States )
- Sucharew, Heidi ( University of Cincinnati , Cincinnati , Ohio , United States )
- Haverbusch, Mary ( University of Cincinnati , Cincinnati , Ohio , United States )
- Kissela, Brett ( University of Cincinnati , Cincinnati , Ohio , United States )
- Broderick, Joseph ( University of Cincinnati , Cincinnati , Ohio , United States )
- Robinson, David ( University of Cincinnati , Cincinnati , Ohio , United States )
- Kleindorfer, Dawn ( Michigan Medicine , Ann Arbor , Michigan , United States )
- Ferioli, Simona ( University of Cincinnati , Cincinnati , Ohio , United States )
- Mackey, Jason ( Indiana University , Indianapolis , Indiana , United States )
- Woo, Daniel ( University of Cincinnati , Cincinnati , Ohio , United States )
- Delosrioslarosa, Felipe ( Baptist Health South Florida , Miami , Florida , United States )
Meeting Info:
Session Info:
Risk Factors and Prevention Posters I
Wednesday, 02/05/2025 , 07:00PM - 07:30PM
Poster Abstract Session
More abstracts on this topic:
Chen Jun-min, Shi Guang, Yu Lulu, Shan Wei, Zhang Xiangjian, Wang Qun
A Community-Based Intervention to Improve Cardiovascular Health Understanding in the Dallas-Fort Worth South Asian CommunityDeo Parminder, Rohatgi Anand, Sharma Parul, Sathyamoorthy Mohanakrishnan
More abstracts from these authors:
Robinson David, Wright Shea, Stamm Brian, Nobel Lisa, Aziz Yasmin, Slavin Sabreena, Walsh Kyle, Delosrioslarosa Felipe, Woo Daniel, Ferioli Simona, Kleindorfer Dawn, Sucharew Heidi, Kissela Brett, Ding Lili, Haverbusch Mary, Stanton Robert, Madsen Tracy, Menzies Lauren, Laporta Joseph, Wechsler Paul
Temporal Trends and Predictors of Door-in-Door-out Times for Interhospital Stroke Transfers in the Greater Cincinnati Northern Kentucky Stroke StudyStamm Brian, Woo Daniel, Wechsler Paul, Menzies Lauren, Wright Shea, Nobel Lisa, Slavin Sabreena, Walsh Kyle, Delosrioslarosa Felipe, Mackey Jason, Mistry Eva, Royan Regina, Demel Stacie, Coleman Elisheva, Jasne Adam, Star Michael, Martini Sharyl, Adeoye Opeolu, Flaherty Matthew, Khatri Pooja, Madsen Tracy, Broderick Joseph, Ding Lili, Kissela Brett, Kleindorfer Dawn, Robinson David, Haverbusch Mary, Ferioli Simona, Stanton Robert, Aziz Yasmin, Laporta Joseph
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.