Final ID: WMP5
Comparative analysis of methods to derive number needed to treat over the entire range of global disability on the modified Rankin Scale
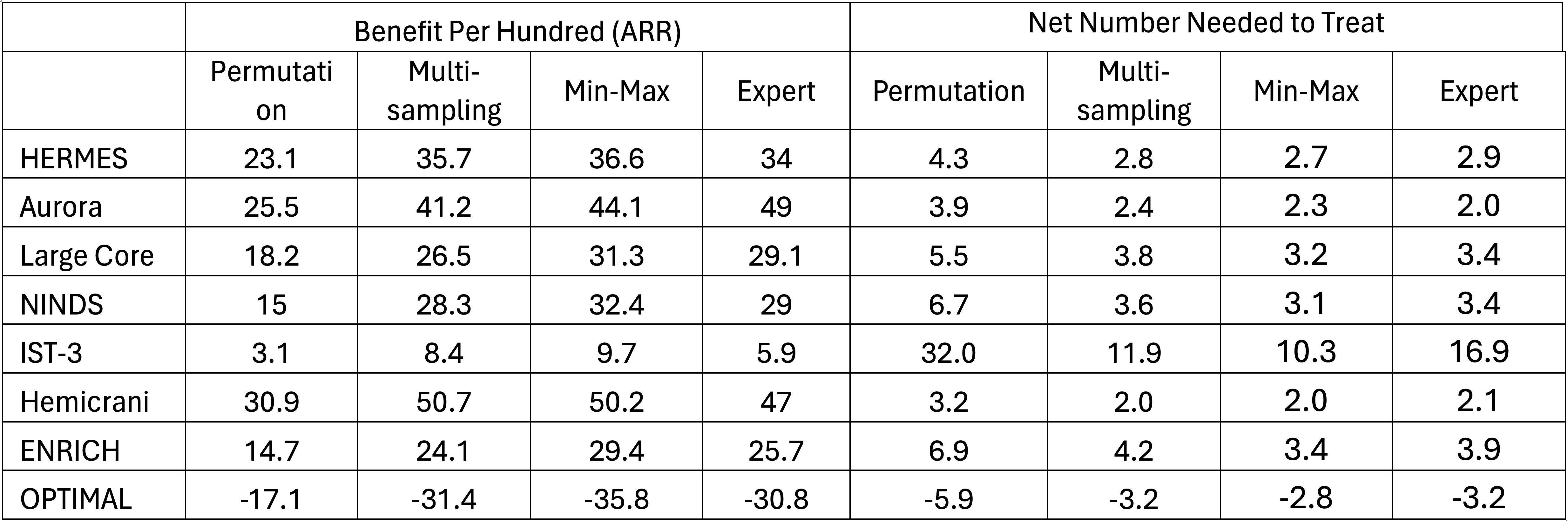
Methods: NNT was calculated for eight stroke clinical rials/meta-analyses purposively selected to include small, moderate and large treatment effects and diverse treatment types, including thrombolysis (NINDS tPA, IST3), thrombectomy (HERMES, AURORA, Large Core EVT), surgery for AIS (Hemicraniectomy), surgery for ICH (ENRICH), and blood pressure control (OPTIMAL BP). We derived benefit per hundred treated (BPH, same as absolute risk reduction) and NNT using four Permutation Methods (CMH test with tied pairs ignored, divided in half, assigned by NIHSS outcome, and by better than model expectations) and three Joint Outcome Table Methods (Multi-sampling, Min-Max, and Expert Panel).
Results: The net BPH and NNT values derived for each trial using each method are shown in the Table. Overall, the magnitude of treatment effect estimates rendered by Permutation methods were substantially lower than by Joint Outcome methods Table methods. In 4 of 8 trials, Permutation method estimates were lower than dichotomous estimates – a biologic impossibility. Among the automatic Joint Outcome Table Methods, the repetitive Multi-sampling technique yielded mildly lower estimates than the algorithmic Min-Max technique.
Conclusions: Permutation (Cochran–Mantel–Haenszel) ordinal NNT derivation methods systematically underestimate treatment benefit magnitude due to bias towards the null from calculation-related nondifferential misclassification. Joint Outcomes Table methods may be preferred for future clinical trials that report net NNT for ordinal outcomes.
More abstracts on this topic:
Prendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara
A randomized controlled trial of antithrombotic therapy in ischemic stroke patients with non-valvular atrial fibrillation and atherosclerosis: The ATIS-NVAF trialOkazaki Shuhei, Uchida Kazutaka, Asakura Koko, Omae Katsuhiro, Yamamoto Haruko, Hirano Teruyuki, Toyoda Kazunori, Iguchi Yasuyuki, Noguchi Teruo, Okada Yasushi, Kitagawa Kazuo, Tanaka Kanta, Sakai Nobuyuki, Yamagami Hiroshi, Yazawa Yukako, Doijiri Ryosuke, Koga Masatoshi, Ihara Masafumi, Yamamoto Shiro, Kamiyama Kenji, Honda Yuko
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.