Final ID: TP224
Design of Extracranial-Intracranial Bypass Surgery for Symptomatic Chronic Middle Cerebral Artery Occlusion Trial (CMOSS-2)
Aim: We aim to determine the efficacy and safety of EC-IC bypass for patients with hemodynamically impaired MCAO.
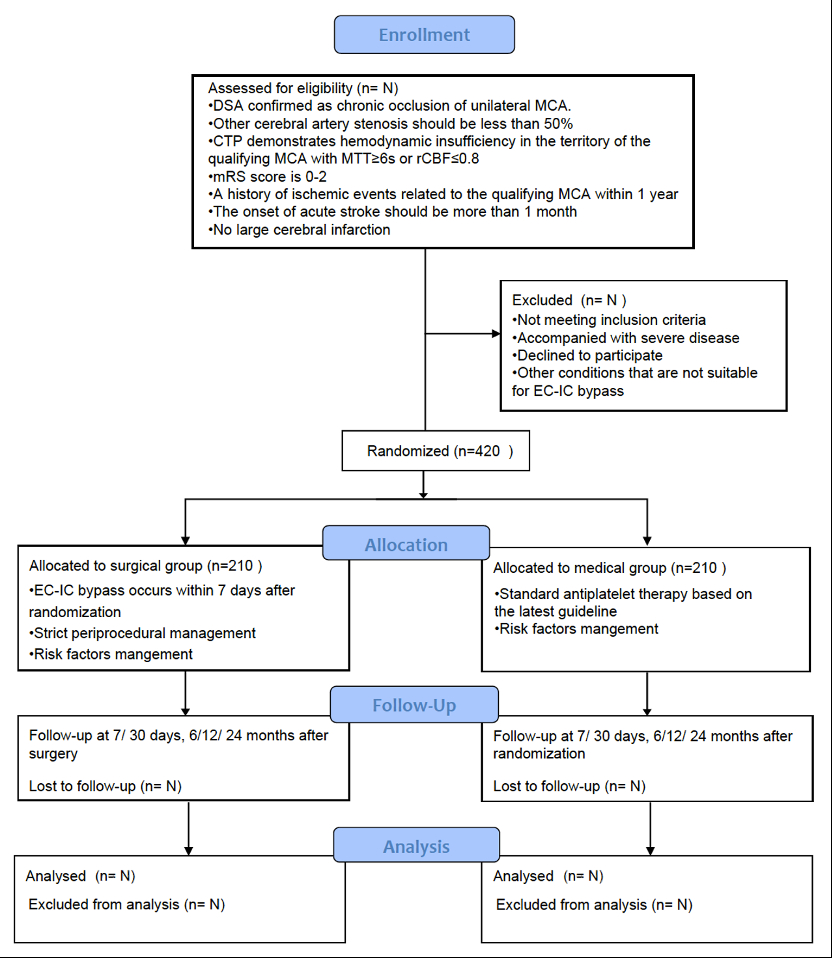
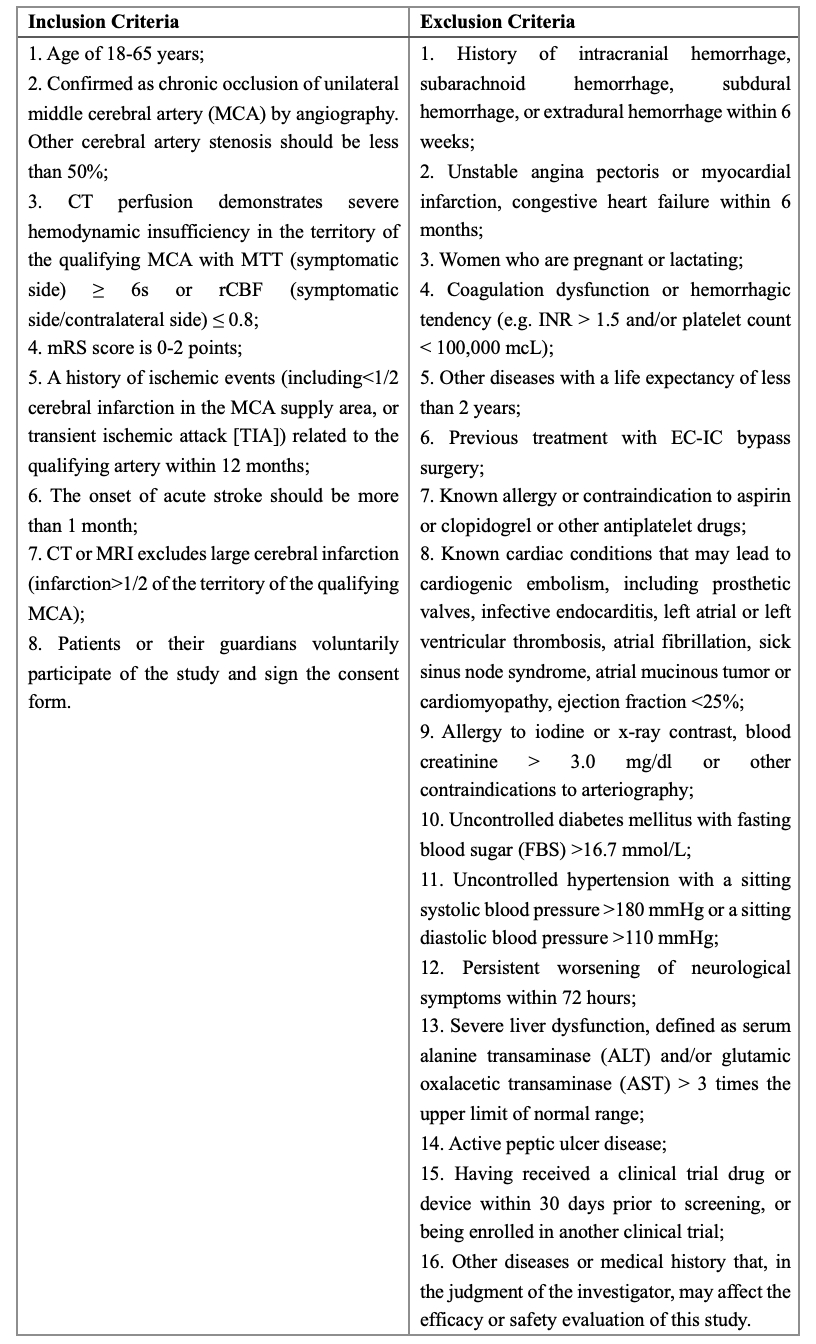
Methods and Design: The CMOSS-2 trial is a government-funded, multi-center, prospective, randomized, open-label, blinded-endpoint (PROBE) trial, which will recruit patients with symptomatic MCAO (parallel design, 1:1 allocation ratio) and severe hemodynamic insufficiency defined by brain CT perfusion (MTT≥6s or rCBF≤0.8). Thirteen high-volume centers are included. Patients will be randomized to EC-IC bypass surgery plus medical treatment or medical treatment alone.
Study outcomes: The primary outcome is the ischemic stroke in territory of qualifying artery within 2 years after randomization. Key secondary outcomes are: Any stroke or death within 30 days after randomization, ischemic stroke in territory of qualifying artery beyond 30 days to 2 years after randomization, any stroke or death within 30 days, or ischemic stroke in territory of qualifying artery beyond 30 days to 2 years after randomization.
Sample size estimates: The estimated difference is 10% in proportions of the primary outcome between the EC-IC bypass group and medical group, which requires 420 (210 per group) participants to provide valid data to achieve a statistical power of 80% and two-tailed alpha of 5% significance after 10% loss to follow-up or early withdrawal.
Discussion: CMOSS-2 study is expected to confirm the effect of EC-IC bypass surgery for symptomatic MCAO patients through the stricter standard for perioperative risk factors management, surgeon screening, and hemodynamic impairment definition.
Trial registration: ClinicalTrials.gov NCT05899582; registered 15 September 2023.
Sponsor: Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (ZLRK202320).
More abstracts on this topic:
Hussain Zaib, Harry Tamunotonye, Michos Erin, Milller Hailey, Juraschek Stephen, Turkson-ocran Ruth-alma, Lahey Timothy, Feng Yuanyuan, Plante Timothy
A Mast Cell-Specific Receptor Mediates Post-Stroke Brain Inflammation Via a Dural-Brain AxisKothari Ruchita, Caplan Justin, Gonzalez L. Fernando, Jackson Christopher, Bettegowda Chetan, Huang Judy, Koehler Raymond, Tamargo Rafael, Xu Risheng, Dong Xinzhong, Abdulrahim Mostafa, Oh Hyun Jong, Capuzzi Daniel, Nair Sumil, Zhang Yaowu, Limjunyawong Nathachit, Saini Sarbjit, Kim Jennifer
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.