Final ID: TMP96
The Use of Direct Oral Anticoagulants in Pediatric Arterial Ischemic Stroke
Methods: A retrospective chart review was approved by Cook Children’s Medical Center (CCMC) institutional review board. Patients (0 to <18 years) treated at CCMC for acute AIS that received rivaroxaban between June 21, 2021-April 1, 2024 were reviewed. Patients treated for concomitant venous thrombosis and/or without follow up imaging were excluded. Data was de-identified and SAS 8.3 was used for analysis. Given small sample, data were summarized using descriptive statistics.
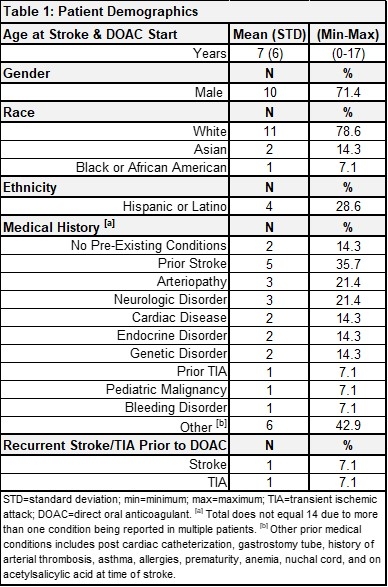
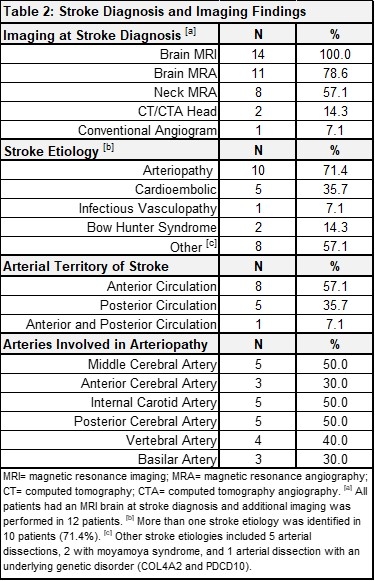
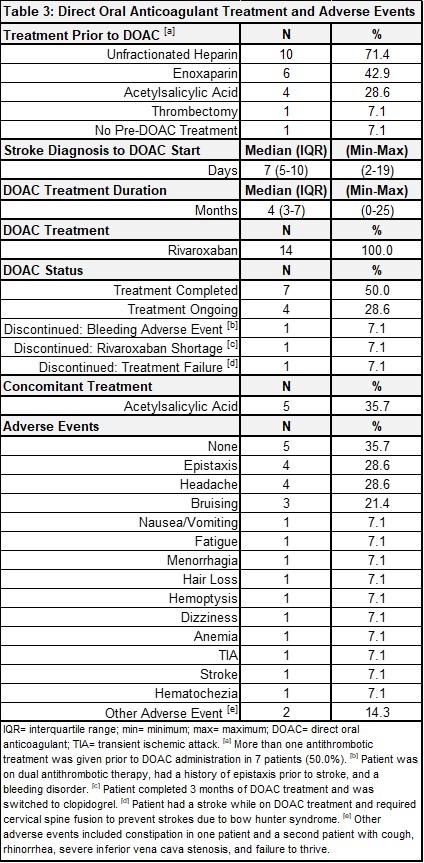
Results: Thirty-two patients were reviewed and 14 met eligibility criteria. Patient demographics and medical history are summarized in Table 1. All had acute AIS confirmed via brain magnetic resonance imaging (MRI), with most strokes affecting the anterior circulation (8, 57.1%) and with arteriopathy as the most common stroke etiology (10, 71.4%) (Table 2). Thirteen (92.9%) received antithrombotic therapy (heparin, enoxaparin, acetylsalicylic acid) prior to DOAC initiation and all 14 were given rivaroxaban afterwards. At the time of data analysis, patients had a median of 4 months of DOAC therapy (interquartile range: 3-7). Four patients remain on treatment (28.6%), 7 completed treatment (50.0%), 1 stopped due to the rivaroxaban shortage (7.1%), 1 discontinued due to bleeding complications (concomitant antiplatelet therapy) (7.1%), and 1 discontinued due to treatment failure (7.1%). All other adverse events were minor not requiring treatment discontinuation (Table 3).
Conclusion: In this study, 14 pediatric AIS patients were treated with rivaroxaban. Only one had it discontinued for patient safety but concomitant therapy with acetylsalicylic acid, made rivaroxaban causality inconclusive. The only treatment failure in our series was a patient with bow hunter syndrome. Even though this study suggests rivaroxaban might be a safe and effective therapeutic option in pediatric AIS, additional research is needed.
More abstracts on this topic:
Penckofer Mary, Salehi Omran Setareh, Seiffge David, Arnold Marcel, Marialuisa Zedde, Zubair Adeel, Marto Joao Pedro, Ghannam Malik, Engelter Stefan, Traenka Christopher, Mac Grory Brian, Shu Liqi, Kam Wayneho, Elnazeir Marwa, Romoli Michele, Saleh Velez Faddi, Siegler James, Strelecky Lukas, Yaghi Shadi, Henninger Nils, Muppa Jayachandra, Bakradze Ekaterina, Heldner Mirjam, Katheryna Antonenko
Acute Stroke Characteristics, Treatment and Outcomes in Children on Mechanical Circulatory SupportCheronis Chrisoula, Jackson Karla, Mayne Elizabeth, Teeyagura Prathyusha, Lee Sarah
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.