Final ID: WP171
Antithrombotic Therapy for Secondary Stroke Prevention in Patients with Severe Chronic Kidney Disease and Atrial Fibrillation
Abstract Body: Background: The prevalence of renal disease is increasing in the U.S. Renal dysfunction increases risk of atrial fibrillation, ischemic stroke and systemic bleeding. Lack of randomized trial data in this population has led to conflicting recommendations on management. Our objective was to review a decade of practice in utilization of antithrombotics including oral anticoagulants (OACs) for secondary stroke prevention in patients with severe renal dysfunction.
Methods: We analyzed all ischemic stroke patients with atrial fibrillation and impaired renal function (creatinine clearance < 30) who were discharged on antithrombotics in the Get with the Guidelines- Stroke registry from Jan 2013 – Dec 2023. Subjects with other indication for anticoagulation such as venous thromboembolism, pulmonary embolism, and prosthetic valve were excluded. For the analysis, subjects were categorized as advanced chronic kidney disease (CKD, CrCl 15-30) and end stage renal disease (ESRD, CrCl<15).
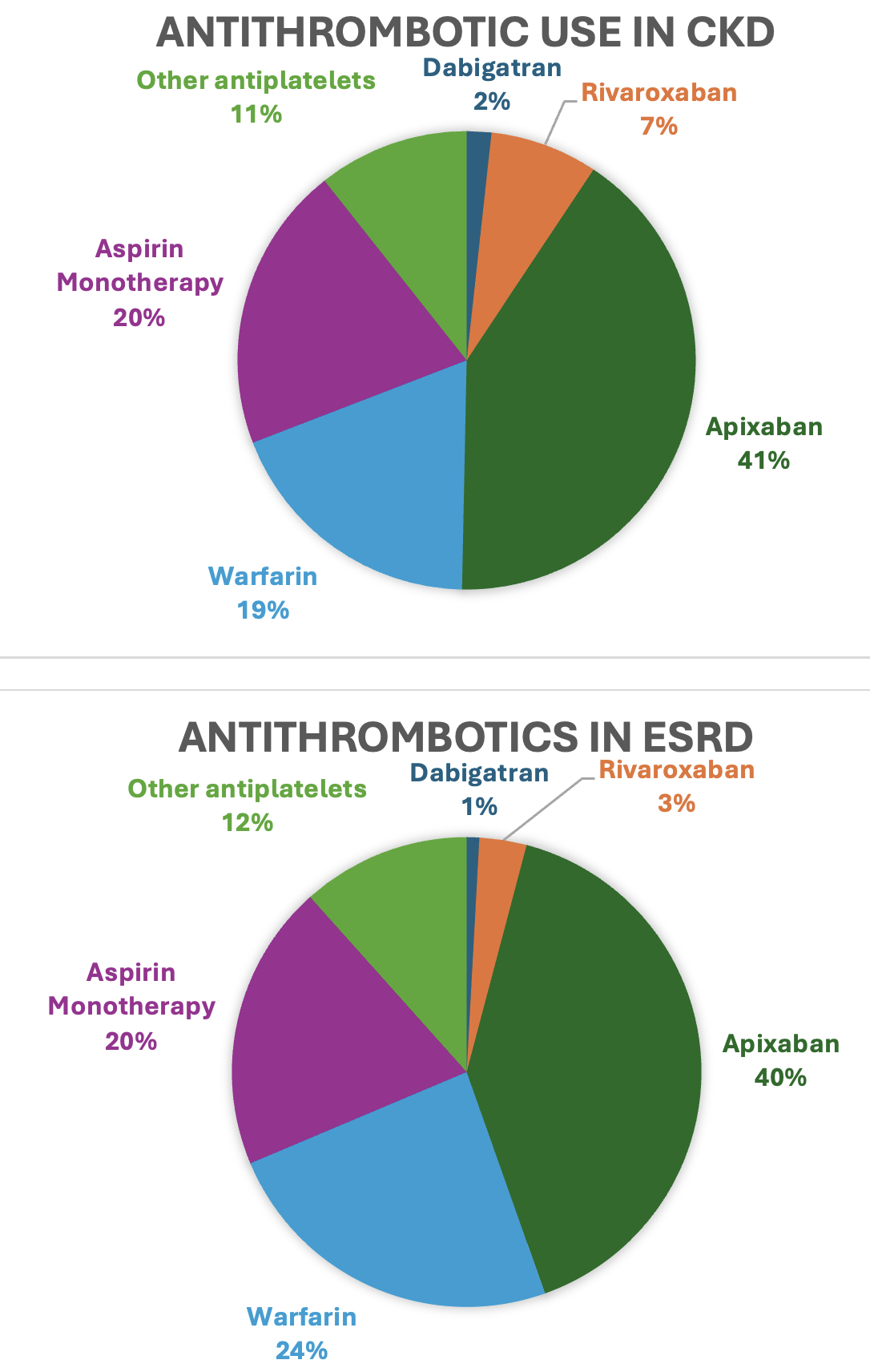
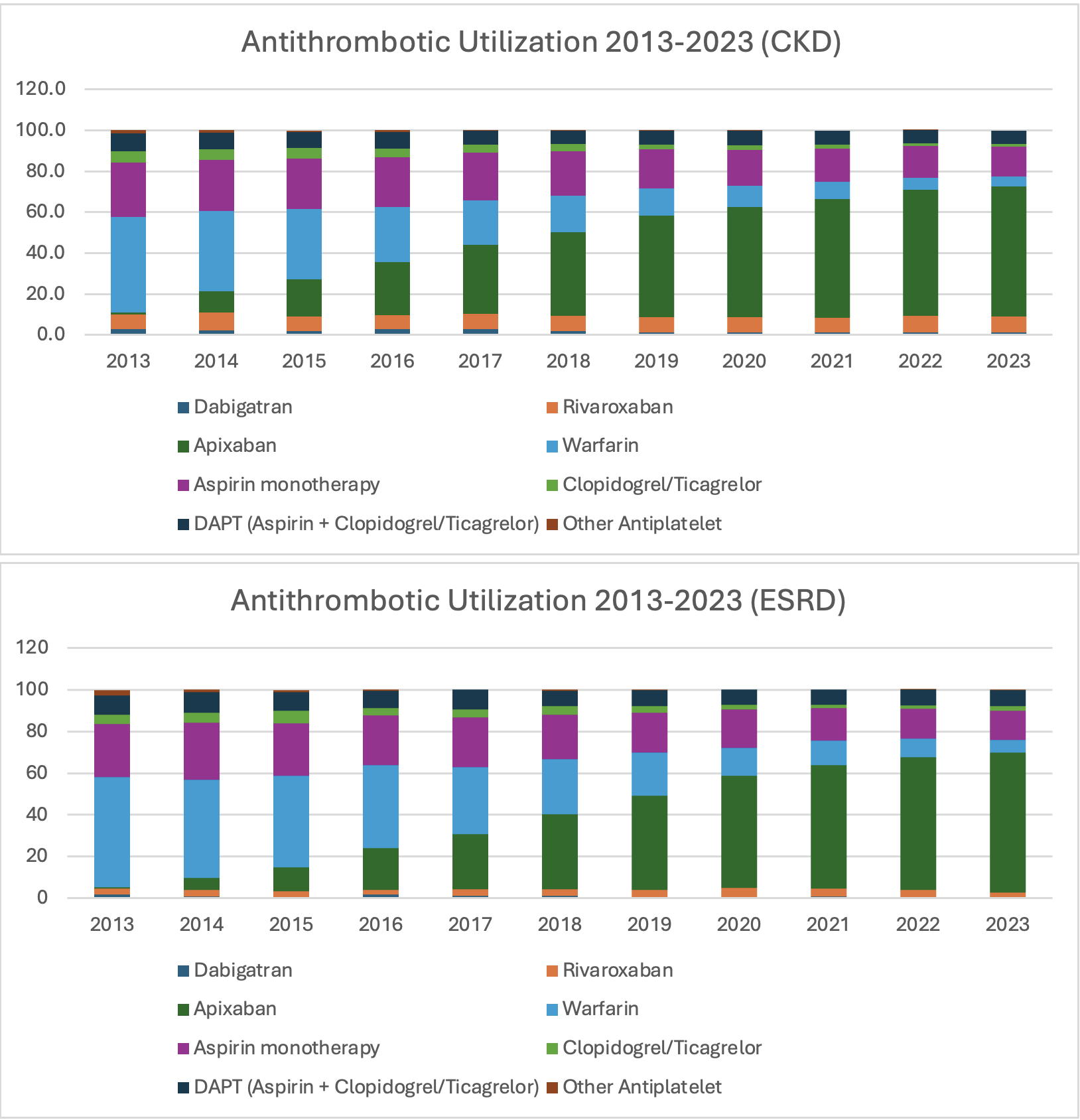
Results: Of 67,209 patients meeting inclusion criteria, 55,501 (82.6%) were classified as CKD and 11,708 (17.4%) as ESRD. Apixaban was the most utilized antithrombotic in both groups (41% in CKD and 40% in ESRD) followed by warfarin in the ESRD group (24%) and aspirin monotherapy (20%) in the CKD group. Among those on apixaban 5mg dose, 24.5% met criteria for the lower 2.5mg dose, yet were treated with the standard dose. Many subjects were discharged on the same oral anticoagulant they were taking at admission: warfarin 26%, apixaban 85%, rivaroxaban 33%, and dabigatran 58%. From 2013 to 2023, Apixaban utilization increased the most in both groups from 1.1% to 63.5% in the CKD group and 0.6% to 66.9% in the ESRD group. Aspirin monotherapy decreased from 27% to 14% in the CKD group and 25% to 14% in the ESRD group.
Conclusions: Trends in OAC utilization show increasing uptake in use of OACs, particularly apixaban, over antiplatelets in the renal dysfunction population. More data is needed to determine the risk/benefit of DOAC vs Vitamin K antagonists, the role of antiplatelets, the inappropriate dosing of DOACs and the management of OAC “failure” in renal patients.
Methods: We analyzed all ischemic stroke patients with atrial fibrillation and impaired renal function (creatinine clearance < 30) who were discharged on antithrombotics in the Get with the Guidelines- Stroke registry from Jan 2013 – Dec 2023. Subjects with other indication for anticoagulation such as venous thromboembolism, pulmonary embolism, and prosthetic valve were excluded. For the analysis, subjects were categorized as advanced chronic kidney disease (CKD, CrCl 15-30) and end stage renal disease (ESRD, CrCl<15).
Results: Of 67,209 patients meeting inclusion criteria, 55,501 (82.6%) were classified as CKD and 11,708 (17.4%) as ESRD. Apixaban was the most utilized antithrombotic in both groups (41% in CKD and 40% in ESRD) followed by warfarin in the ESRD group (24%) and aspirin monotherapy (20%) in the CKD group. Among those on apixaban 5mg dose, 24.5% met criteria for the lower 2.5mg dose, yet were treated with the standard dose. Many subjects were discharged on the same oral anticoagulant they were taking at admission: warfarin 26%, apixaban 85%, rivaroxaban 33%, and dabigatran 58%. From 2013 to 2023, Apixaban utilization increased the most in both groups from 1.1% to 63.5% in the CKD group and 0.6% to 66.9% in the ESRD group. Aspirin monotherapy decreased from 27% to 14% in the CKD group and 25% to 14% in the ESRD group.
Conclusions: Trends in OAC utilization show increasing uptake in use of OACs, particularly apixaban, over antiplatelets in the renal dysfunction population. More data is needed to determine the risk/benefit of DOAC vs Vitamin K antagonists, the role of antiplatelets, the inappropriate dosing of DOACs and the management of OAC “failure” in renal patients.
More abstracts on this topic:
Association of Pre-operative Neutrophil to Lymphocyte Ratio (NLR) and Post-operative AKI in Patients Undergoing CABG: A Meta-Analysis
Patel Bhavin, Lapsiwala Boney, Jariwala Prayag, Suresh Aditya, Vanani Samir, Patel Shaurya, Rajani Aayushi, Desai Rupak
A Hidden Threat: Trazodone-Induced Warfarin Failure Leading to Acute Mechanical Aortic Valve ThrombosisVogt Cody, Malik Saad, Pfirman Kristopher
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)