Final ID: WP217
Risks and Benefits of Lecanemab and Anticoagulants: Results from a Simulation Model
Abstract Body: Background: In CLARITY-AD, lecanemab slowed cognitive decline but increased intracranial hemorrhages (ICHs), particularly with concurrent anticoagulant use. The Alzheimer’s Association’s expert guidance is to avoid co-prescribing; however, CMS and FDA do not restrict or warn against it. We used a microsimulation model to quantify the potential benefits and harms of co-prescribing lecanemab and apixaban in people with atrial fibrillation (AF) experiencing mild cognitive impairment or early Alzheimer’s.
Methods: We developed a microsimulation model to estimate the health and cognition-related quality of life among persons 65-90 years with AF and cognitive impairment. We compared 4 strategies over 18 months in a cohort of 100,000 people: apixaban alone, lecanemab and apixaban, lecanemab alone, and neither. The model was populated with the Health and Retirement Study-AF cohort. Monthly model outcomes included ICH, ischemic stroke, cognitive impairment, quality-adjusted life months (QALMs), and survival. Increased ICH risk was a key input: a trial-reported 2.02-fold increase for lecanemab alone, a 1.84-fold increase for apixaban alone (anticoagulant literature), and a trial-reported 9.92-fold increase for lecanemab and anticoagulants together. We assigned quality-of-life estimates and mortality rates for people with cognitive impairment, stroke, and ICH. Background mortality rates increased with cognitive decline and following a stroke or ICH event.
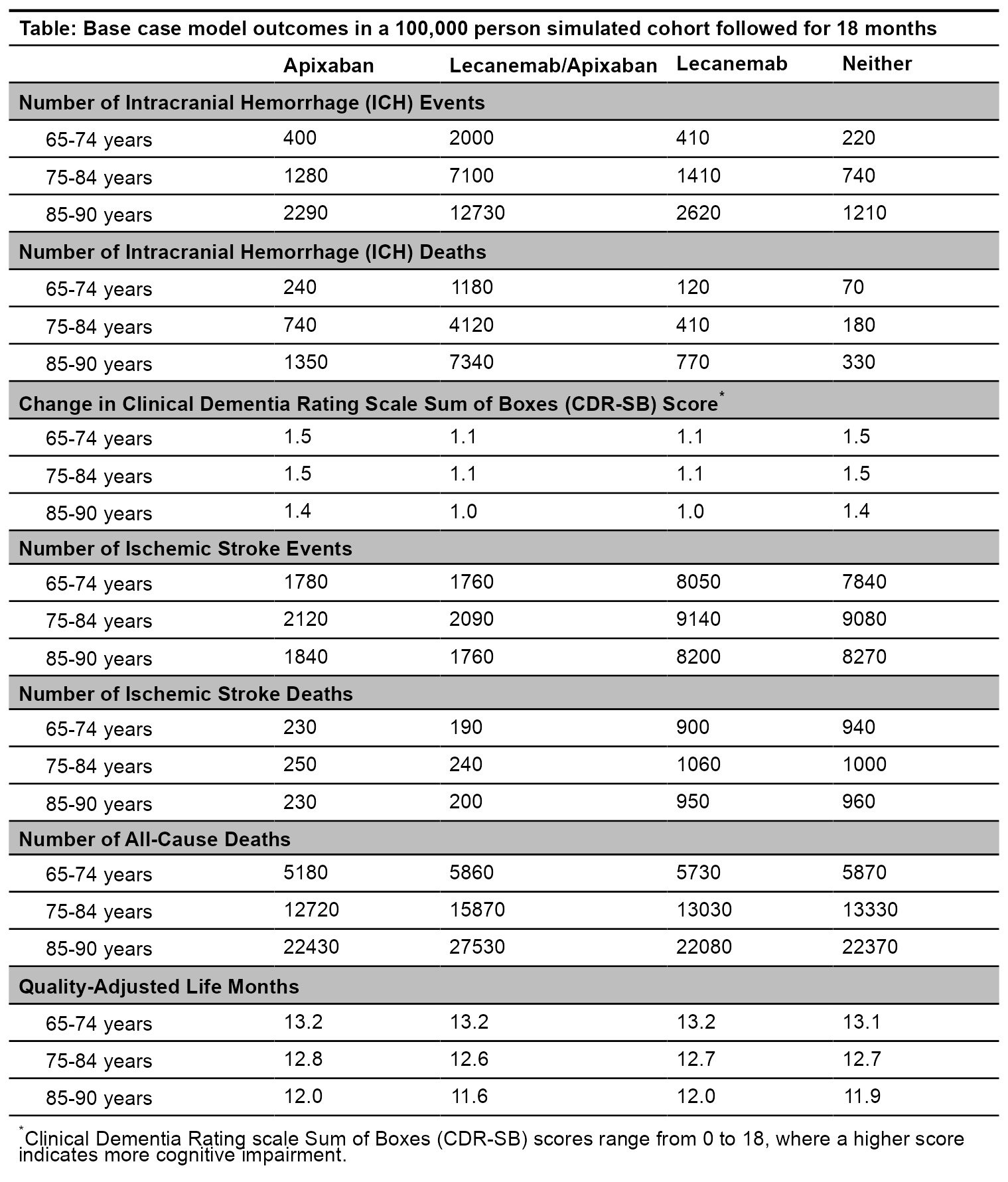
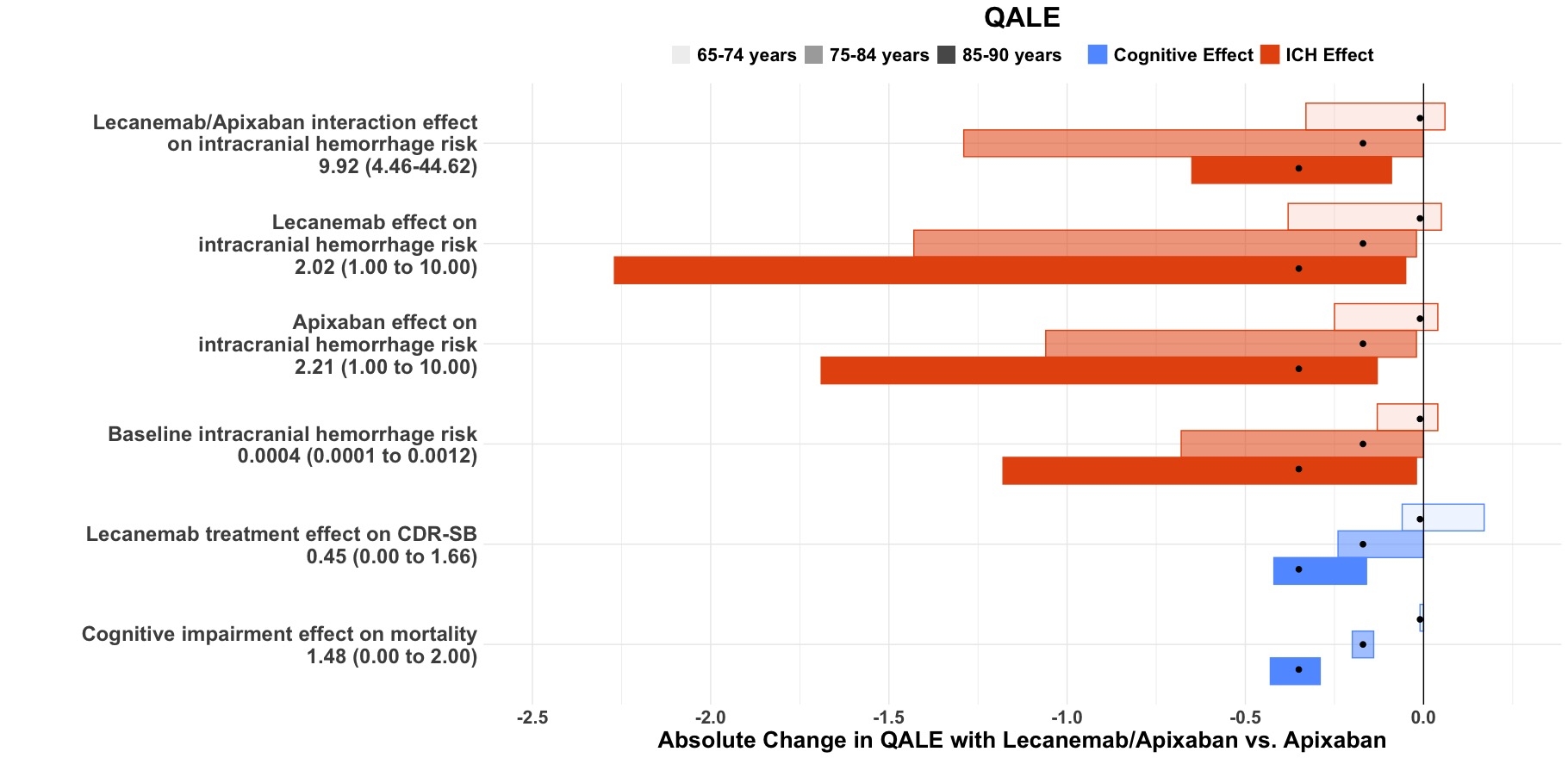
Results: For ages 65-74, apixaban alone and lecanemab added to apixaban produced a similar net benefit (13.2 QALM each, Table). Over 100,000 simulated persons aged 65-74 years, adding lecanemab to apixaban would result in greater ICH events (2000 vs. 400) and all-cause deaths (5860 vs. 5180) and slower cognitive decline (mean change in CDR-SB 1.11 vs. 1.53). One-way sensitivity analyses show that the net benefit for people aged 65-74 years is sensitive to the determinants of ICH—lecanemab/anticoagulant interaction, lecanemab effect on ICH, apixaban effect on ICH, baseline ICH risk—and lecanemab effect on CDR-SB (Figure). Apixaban alone was preferred for people 75 years and older.
Conclusion: The model-based results suggest equipoise between apixaban alone and lecanemab with apixaban for people with cognitive impairment and AF aged 65-74 years. Improving lecanemab efficacy or reducing its effect on ICH could produce a net benefit for this age group. For people 75 and older, apixaban alone would be preferred.
Methods: We developed a microsimulation model to estimate the health and cognition-related quality of life among persons 65-90 years with AF and cognitive impairment. We compared 4 strategies over 18 months in a cohort of 100,000 people: apixaban alone, lecanemab and apixaban, lecanemab alone, and neither. The model was populated with the Health and Retirement Study-AF cohort. Monthly model outcomes included ICH, ischemic stroke, cognitive impairment, quality-adjusted life months (QALMs), and survival. Increased ICH risk was a key input: a trial-reported 2.02-fold increase for lecanemab alone, a 1.84-fold increase for apixaban alone (anticoagulant literature), and a trial-reported 9.92-fold increase for lecanemab and anticoagulants together. We assigned quality-of-life estimates and mortality rates for people with cognitive impairment, stroke, and ICH. Background mortality rates increased with cognitive decline and following a stroke or ICH event.
Results: For ages 65-74, apixaban alone and lecanemab added to apixaban produced a similar net benefit (13.2 QALM each, Table). Over 100,000 simulated persons aged 65-74 years, adding lecanemab to apixaban would result in greater ICH events (2000 vs. 400) and all-cause deaths (5860 vs. 5180) and slower cognitive decline (mean change in CDR-SB 1.11 vs. 1.53). One-way sensitivity analyses show that the net benefit for people aged 65-74 years is sensitive to the determinants of ICH—lecanemab/anticoagulant interaction, lecanemab effect on ICH, apixaban effect on ICH, baseline ICH risk—and lecanemab effect on CDR-SB (Figure). Apixaban alone was preferred for people 75 years and older.
Conclusion: The model-based results suggest equipoise between apixaban alone and lecanemab with apixaban for people with cognitive impairment and AF aged 65-74 years. Improving lecanemab efficacy or reducing its effect on ICH could produce a net benefit for this age group. For people 75 and older, apixaban alone would be preferred.
More abstracts on this topic:
Age and Sex Multiplicatively Moderate the Association of Daily Sedentary Time with Depressive Symptoms in Rural Patients with Cardiovascular Diseases
Kang Junghee, Moser Debra, Cha Geunyeong, Lin Chin-yen, Wu Jia-rong, Okoli Chizimuzo, Latimer Abigail, Lennie Terry, Biddle Martha, Chung Misook
Age and White Matter Injury due to Cerebral Small Vessel Disease are Synergistically Associated with Impaired Neurovascular Coupling.Yang Sheng, Webb Alastair
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)