Final ID: Mo4046
Elevated International Normalized Ratio Is Associated with Severity of Intracerebral Hemorrhage for Patients Taking Direct Oral Anticoagulants
Research Questions/Hypothesis: To determine whether INR is associated with increased ICH severity in patients with recent exposure to a DOAC.
Methods/Approach: We included patients taking Apixaban or Rivaroxaban prior to acute presentation with ICH to hospitals participating in the Get With the Guidelines - Stroke registry, a nationwide quality improvement registry. The primary exposure was INR, which we modeled as a continuous variable with restricted cubic splines. The primary endpoint was stroke severity on presentation, measured by the National Institutes of Health Stroke Scale (NIHSS). Secondary endpoints included Glasgow Coma Scale (GCS) <=8 on admission, in-hospital mortality, composite of in-hospital mortality and discharge to hospice, and modified Rankin Scale (mRS) at discharge. We adjusted for key confounding factors, specifically age, sex, race/ethnicity, insurance status, and medical history, while taking into account within-hospital clustering.
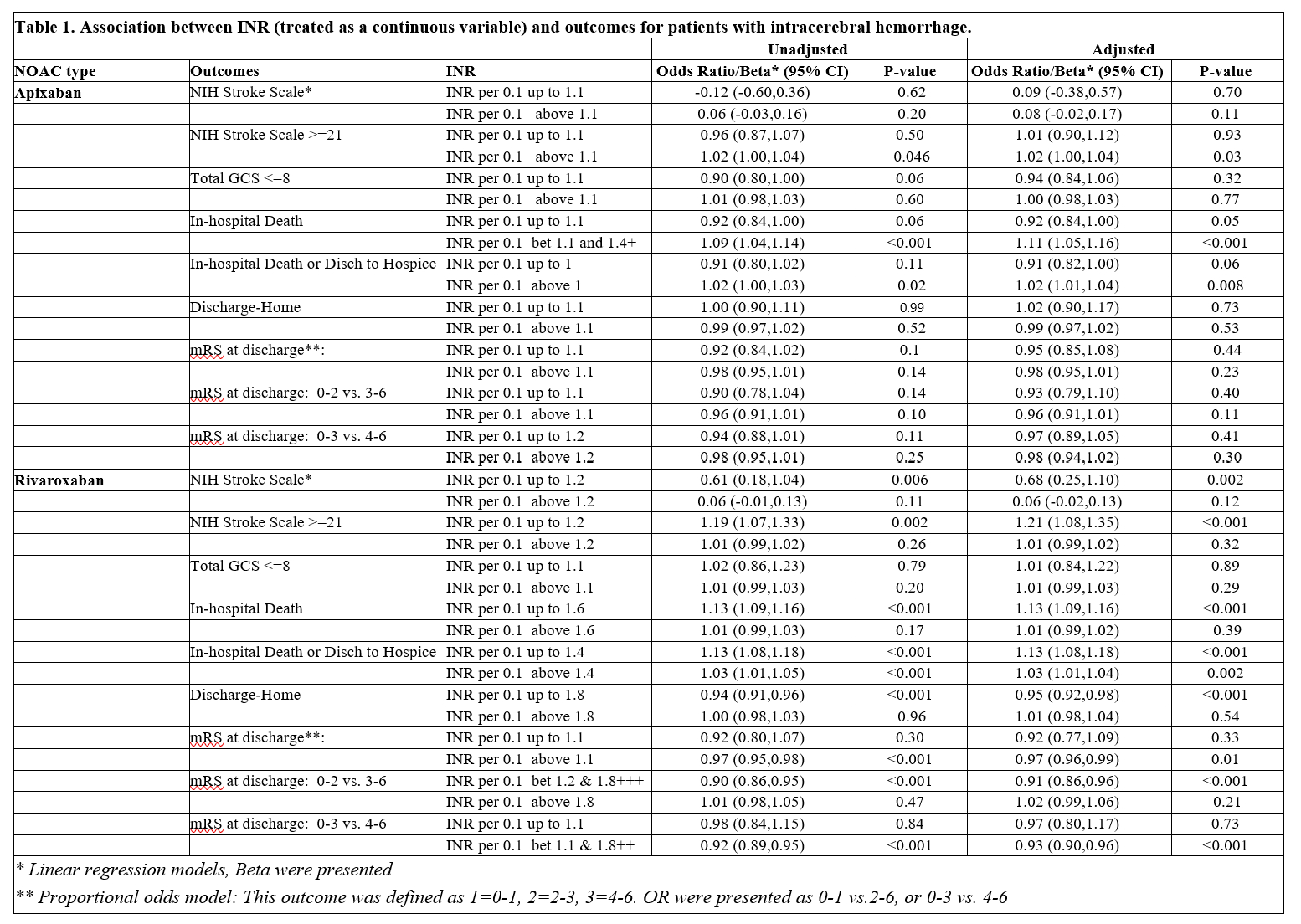
Results/Data: In total, 13,251 patients were included of whom 7,545 were exposed to apixaban and 5,706 were exposed to rivaroxaban. As shown in Table 1, for patients taking Apixaban, elevated INR was associated with increased likelihood of NIHSS >=21 (aOR 1.02 [95% CI 1.00-1.04, p=0.03] per 0.1 above 1.1) and in-hospital mortality (aOR 1.11 [95% CI 1.05-1.16, p<.001) per 0.1 above 1.1). For patients taking Rivaroxaban, similar trends were seen (aOR 1.21 [95% CI 1.08-1.35, p<.001] per 0.1 up to 1.2 for NIHSS >=21, aOR 1.13, [95% CI 1.09-1.16, p<.001] per 0.1 up to 1.6, for in-hospital mortality).
Conclusion(s): Elevated INR at admission is associated with worsened ICH severity for patients taking Apixaban or Rivaroxaban.
- Lusk, Jay ( Duke University , Durham , North Carolina , United States )
- Matsouaka, Roland ( Duke University , Durham , North Carolina , United States )
- Xian, Ying ( UTSW , Dallas , Texas , United States )
- Sun, Jie-lena ( Duke University Clinical Research Institute , Durham , North Carolina , United States )
- Mac Grory, Brian ( Duke University Clinical Research Institute , Durham , North Carolina , United States )
- Schwamm, Lee ( Yale School of Medicine , New Haven , Connecticut , United States )
- Smith, Eric ( UNIVERSITY OF CALGARY , Calgary , Alberta , Canada )
- Fonarow, Gregg ( UCLA MEDICAL CENTER , Los Angeles , California , United States )
- Bhatt, Deepak ( Mount Sinai , New York , New York , United States )
- Saver, Jeffrey ( GEFFEN SCHOOL OF MEDICINE AT UCLA , Los Angeles , California , United States )
- Reeves, Mathew ( MICHIGAN STATE UNIVERSITY , East Lansi , Michigan , United States )
Meeting Info:
Session Info:
Pioneering Approaches to Arterial and Emergency Conditions
Monday, 11/18/2024 , 01:30PM - 02:30PM
Abstract Poster Session
More abstracts on this topic:
Ali Md Akkas, Vardaman, Donald, Bolding Chase, Tidwell Harrison, Tyrrell Daniel
Cardiovascular Events in Hospitalized Patients with Malignant Neuroendocrine Tumors: A Nationwide AnalysisPhilip Anil, Banga Akshat, Saeed Muhammad Subhan, Briones-zamora Killen H., Briones-claudett Killen H., Kohli Saksham, Khullar Rohit, George Lina James, Mautong Hans, John Kevin, Varma Revati, Kini Saurav, Khalid Abdullah, Saha Shubhashis, Caputi Zuniga Angelo
More abstracts from these authors:
Lusk Jay, Matsouaka Roland, Xian Ying, Sun Jie-lena, Mac Grory Brian, Schwamm Lee, Smith Eric, Fonarow Gregg, Bhatt Deepak, Saver Jeffrey, Reeves Mathew
Race-Ethnic Specific Hospital Arrival Time of Acute Ischemic Stroke by State in the U.S.Chen Bing Yu, Xian Ying, Uchino Ken, Man Shumei, Sun Jie-lena, Fonarow Gregg, Alhanti Brooke, Mac Grory Brian, Smith Eric, Schwamm Lee, Bhatt Deepak, Saver Jeffrey