Final ID: 6
HRS8179 in Patients with Cerebral Edema after Large Hemispheric Infarction: A Multicenter, Randomized, Double-blinded, Placebo-controlled, Phase 2 Study
Methods: In this multicenter, randomized, double-blinded, placebo-controlled, phase 2 clinical trial (NCT05690711), patients who had a clinical diagnosis of LHI for <10 hours (confirmed by diffusion-weighted image or computed tomography perfusion lesion volume of 80-300 cm3) and National Institutes of Health Stroke Scale (NIHSS) ≥10 were randomized (1:1) to receive HRS8179 or placebo administered as a 0.15 mg intravenous injection followed by a 0.1 mg/h continuous intravenous infusion for 72 hours. The primary endpoint was the change in midline shift from the baseline after 72 hours of treatment.
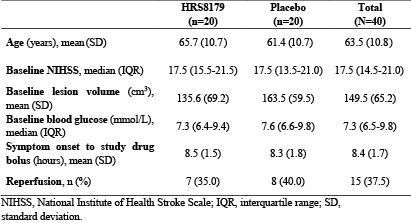
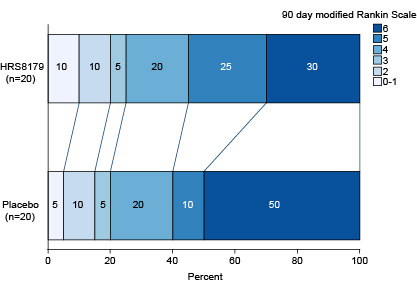
Findings: A total of 40 patients were randomized (20 in the HRS8179 group, 20 in the placebo group). Baseline characteristics were similar between groups (Table). HRS8179-treated patients had a LS mean change in midline shift relative to baseline at 72 hours of 7.92 mm, compared with 9.17 mm in placebo-treated patients (difference, -1.25 mm [95% CI, -5.50 to 3.00]). The 90-day mortality rate in the HRS8179 group and the placebo group were 30.0% (95% CI, 11.9% to 54.3%) and 50.0% (95% CI, 27.2% to 72.8%), respectively. Distribution of modified Rankin Scale scores at 90 days for both groups is shown (Figure). The increase in NIHSS ≥4 points at 72 hours occurred in 5 patients (26.3%) in the HRS8179 group and 7 patients (41.2%) in the placebo group. At 2 weeks of symptom onset, 6 patients (30.0%) in the HRS8179 group and 13 patients (65.0%) in the placebo group experienced brain herniation or death. The most common treatment-related adverse events (TRAEs) in the HRS8179 group were hypoglycemia (7 [35.0%]). The incidence of hypoglycemia with blood glucose levels <3.9 mmol/L was 30.0% and levels <3.0 mmol/L was 15.0%, which were rapidly resolved by intravenous glucose supplementation. No TRAEs led to death.
Interpretation: HRS8179 showed potential efficacy in reducing the severity of cerebral edema in patients with LHI, particularly in lowering the 90-day mortality rate, even in patients without reperfusion, with an acceptable safety profile.
More abstracts on this topic:
Yoon Chung Eun, Kim You Bin, Nam Hyo Suk
A randomized controlled trial of antithrombotic therapy in ischemic stroke patients with non-valvular atrial fibrillation and atherosclerosis: The ATIS-NVAF trialOkazaki Shuhei, Uchida Kazutaka, Asakura Koko, Omae Katsuhiro, Yamamoto Haruko, Hirano Teruyuki, Toyoda Kazunori, Iguchi Yasuyuki, Noguchi Teruo, Okada Yasushi, Kitagawa Kazuo, Tanaka Kanta, Sakai Nobuyuki, Yamagami Hiroshi, Yazawa Yukako, Doijiri Ryosuke, Koga Masatoshi, Ihara Masafumi, Yamamoto Shiro, Kamiyama Kenji, Honda Yuko
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.