Final ID: 44
Minimally Invasive Surgery is Associated with Improved Outcomes Compared to Open Craniotomy with Clot Evacuation after Spontaneous Intracerebral Hemorrhage in the AHA Get With The Guidelines Registry
Abstract Body: Introduction: Data are lacking on outcomes of minimally invasive surgery (MIS), an emerging surgical treatment for spontaneous intracerebral hemorrhage (ICH), compared to conventional open craniotomy with clot evacuation. We therefore sought to evaluate ICH outcomes after MIS vs conventional clot evacuation in a real-world, nationally representative US cohort.
Methods: We performed a retrospective cohort study of patients with ICH from 2011-2021 in the Get With The Guidelines-Stroke registry. The exposure was the type of surgery, classified as open craniotomy with clot evacuation versus MIS (either endoscopic surgical evacuation or stereotactic evacuation with fibrinolytic therapy). The primary outcome was in-hospital mortality, and secondary outcomes included discharge disposition, ambulatory status at discharge, and modified Rankin score at discharge. Using overlap propensity matching, we matched patients with MIS versus open craniotomy on age, sex, race, NIH Stroke Scale, prior antithrombotic therapy, external ventricular drain use, and withdrawal of care. The overlap weighting creates exact balance on the mean of every measured covariate when the propensity score is estimated by logistic regression, and therefore, mimics the attributes of a clinical trial. Each MIS patient was matched with multiple patients who received open craniotomy, based on the propensity score. Logistic regression was used to study the relationship of the type of surgery with outcomes. Pre-specified subgroup analyses were stratified by age, sex, race, NIHSS, EVD use, and annual ICH volume.
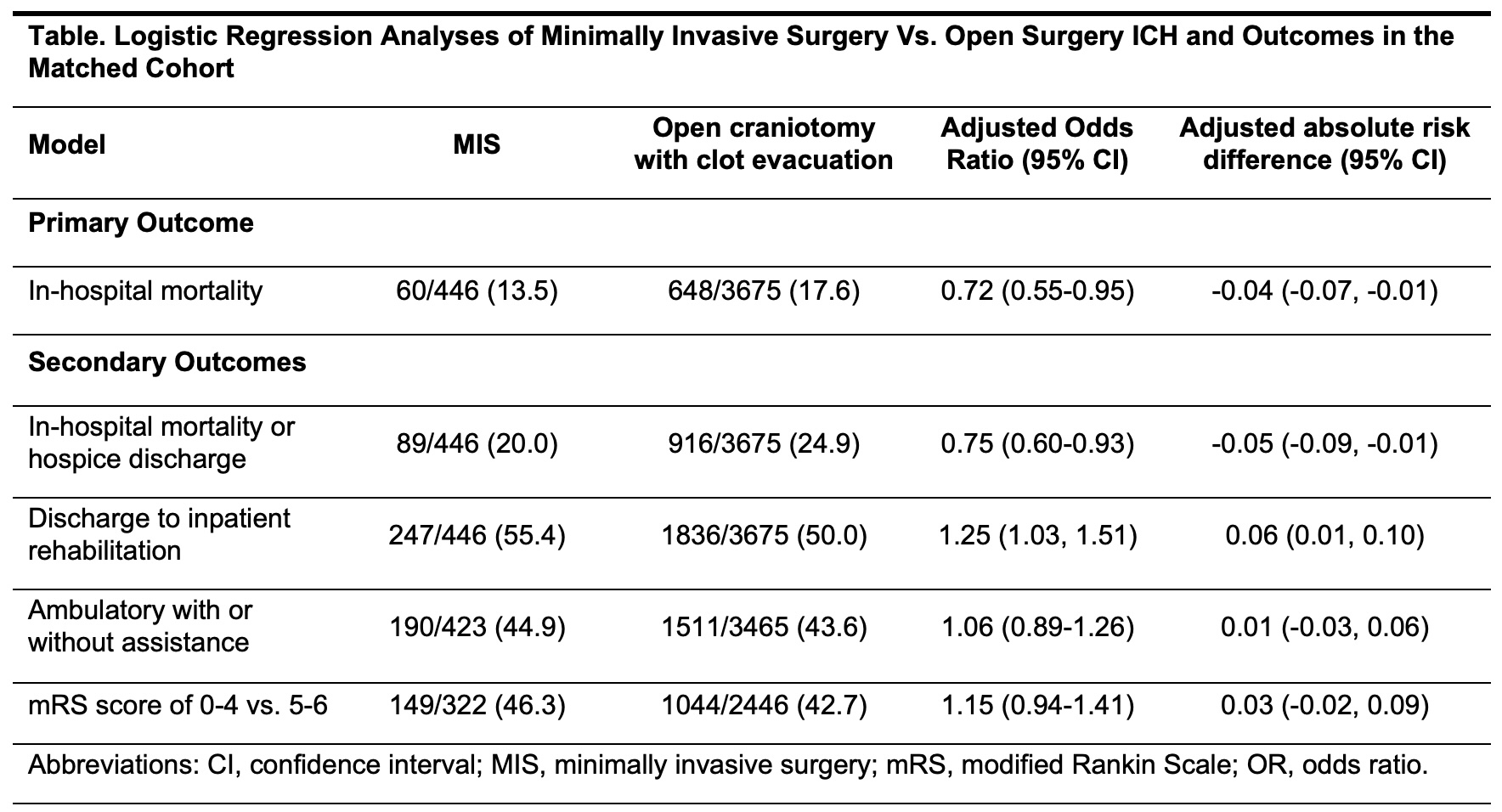
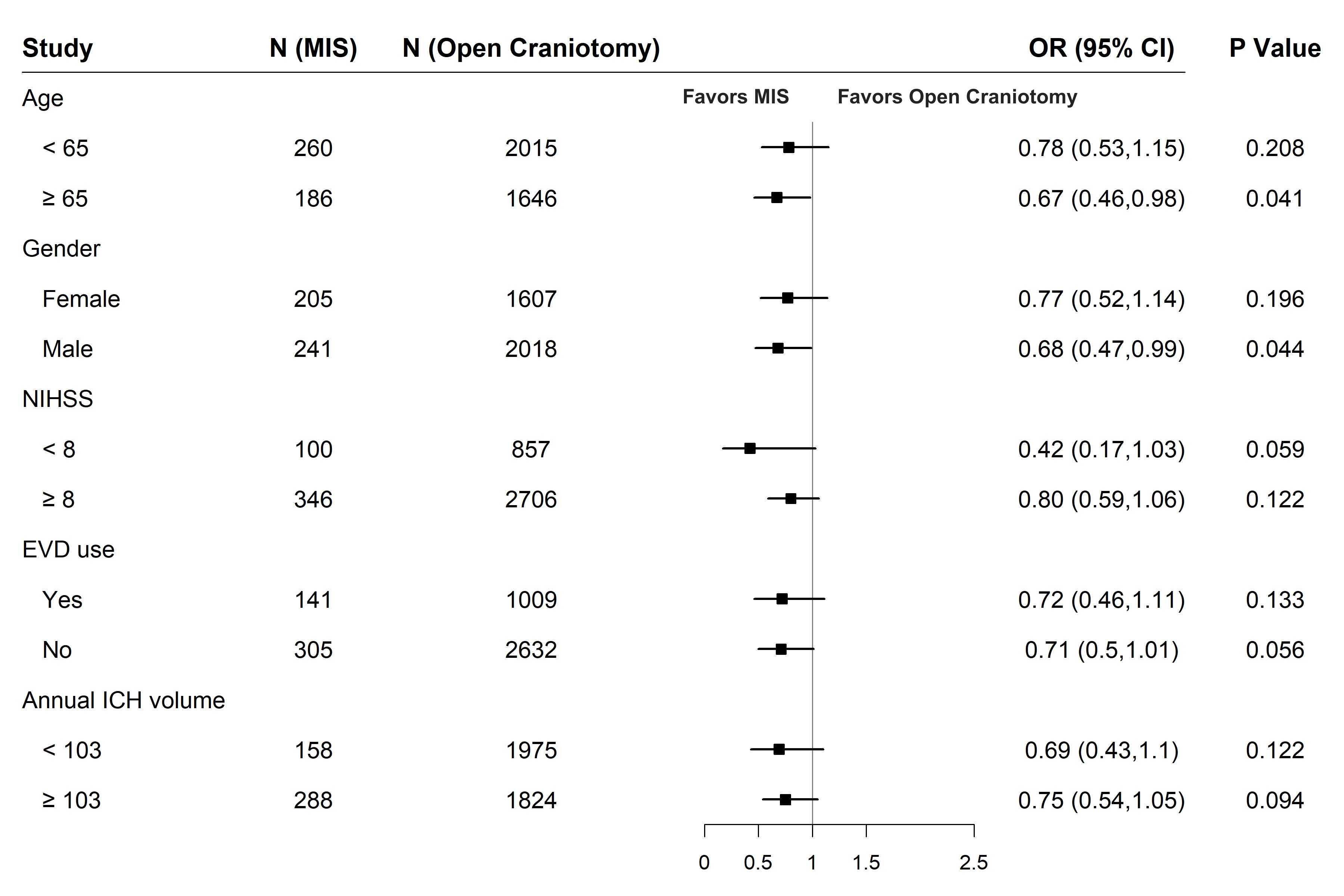
Results: Among 555,964 patients with ICH, MIS was performed in 703 patients (330 had stereotactic surgery and 373 had endoscopic surgery) and open craniotomy was performed in 7067 patients. In the matched cohort, in-hospital deaths occurred in 60/446 (13.5%) with MIS and 648/3675 (17.6%) with open craniotomy. In regression analyses, MIS was associated with lower odds of in-hospital mortality (aOR, 0.7; CI, 0.5-0.9), unfavorable discharge (aOR, 0.7; CI, 0.6-0.9), and higher odds of discharge to rehabilitation (aOR, 1.3; CI, 1.1-1.5), but not with functional outcomes (Table). In pre-specified subgroup analyses, lower mortality was noted with MIS in older patients and men (Figure).
Conclusions: In a large, diverse US cohort of ICH patients, MIS was associated with lower odds of in-hospital mortality and better discharge disposition compared to open craniotomy with clot evacuation.
Methods: We performed a retrospective cohort study of patients with ICH from 2011-2021 in the Get With The Guidelines-Stroke registry. The exposure was the type of surgery, classified as open craniotomy with clot evacuation versus MIS (either endoscopic surgical evacuation or stereotactic evacuation with fibrinolytic therapy). The primary outcome was in-hospital mortality, and secondary outcomes included discharge disposition, ambulatory status at discharge, and modified Rankin score at discharge. Using overlap propensity matching, we matched patients with MIS versus open craniotomy on age, sex, race, NIH Stroke Scale, prior antithrombotic therapy, external ventricular drain use, and withdrawal of care. The overlap weighting creates exact balance on the mean of every measured covariate when the propensity score is estimated by logistic regression, and therefore, mimics the attributes of a clinical trial. Each MIS patient was matched with multiple patients who received open craniotomy, based on the propensity score. Logistic regression was used to study the relationship of the type of surgery with outcomes. Pre-specified subgroup analyses were stratified by age, sex, race, NIHSS, EVD use, and annual ICH volume.
Results: Among 555,964 patients with ICH, MIS was performed in 703 patients (330 had stereotactic surgery and 373 had endoscopic surgery) and open craniotomy was performed in 7067 patients. In the matched cohort, in-hospital deaths occurred in 60/446 (13.5%) with MIS and 648/3675 (17.6%) with open craniotomy. In regression analyses, MIS was associated with lower odds of in-hospital mortality (aOR, 0.7; CI, 0.5-0.9), unfavorable discharge (aOR, 0.7; CI, 0.6-0.9), and higher odds of discharge to rehabilitation (aOR, 1.3; CI, 1.1-1.5), but not with functional outcomes (Table). In pre-specified subgroup analyses, lower mortality was noted with MIS in older patients and men (Figure).
Conclusions: In a large, diverse US cohort of ICH patients, MIS was associated with lower odds of in-hospital mortality and better discharge disposition compared to open craniotomy with clot evacuation.
More abstracts on this topic:
A 50% or Greater Reduction in LDL-Cholesterol Is Associated with Improved Long-Term Outcomes and Lower Health Care Utilization After Myocardial Infarction - a SWEDEHEART study
Reitan Christian, Watanabe Alexandre, Bash Lori, Galvain Thibaut, Arnet Urs, Jernberg Tomas
A Multicenter Observational Study of Bleeding Events in Critically Ill Surgical and Trauma AdolescentsRahman Fahmid, Faustino E. Vincent, Popham Jonathan
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)