Final ID: 156
A Mast Cell-Specific Receptor Mediates Post-Stroke Brain Inflammation Via a Dural-Brain Axis
Abstract Body: Background: Brain inflammation following ischemic stroke exacerbates neuronal injury. Early immune cell infiltration into the brain after ischemia is correlated with increased risk of subsequent stroke and higher three-month mortality. However, direct mechanisms that underly this immune cell recruitment remain unclear, preventing the development of successful therapeutics. Recent work has identified mast cells as early responders in stroke that incite inflammation, yet how these cells are activated remains unknown. We hypothesized that Mrgprb2, a mast cell receptor known to trigger neurogenic inflammation, initiates mast cell activity in stroke, and that inhibition of this receptor can attenuate stroke injury.
Methods: We performed MCA occlusion (MCAO) on wild-type and Mrgprb2-null (Mrgprb2-/-) mice and assessed stroke volume, and behavior. We used flow cytometry and ELISA for immune cell and cytokine profiling to evaluate inflammation. Human stroke patient dura and blood was sampled to assess mast cell activity and to identify potential ligands for Mrgprb2 activation. Lastly, we used osthole, a Mrgprb2 inhibitor, as a post-MCAO treatment to determine if pharmacologic inhibition can attenuate post-stroke deficits in mice.
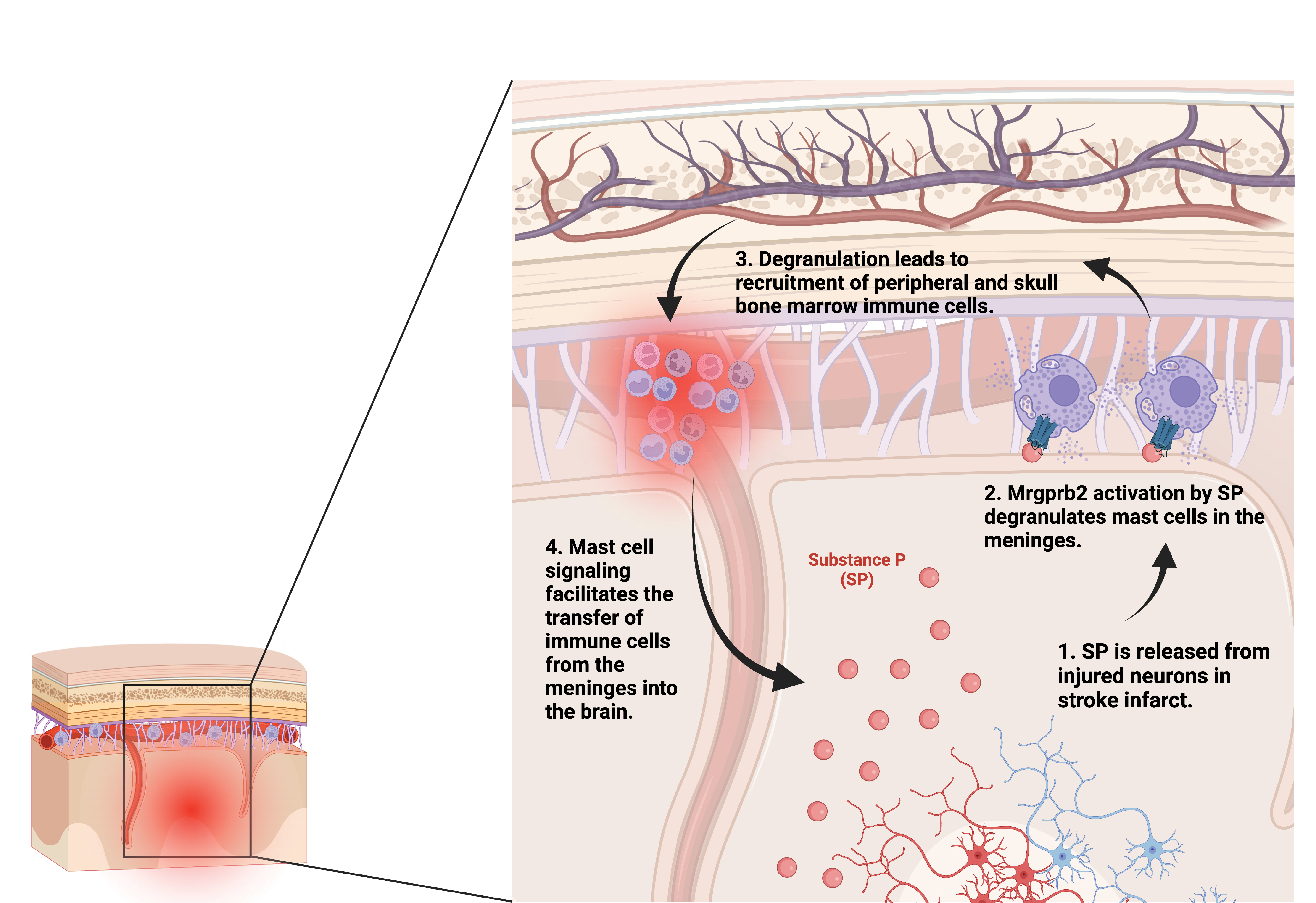
Results: We found that Mrgprb2 is activated in meningeal mast cells after stroke, causing mast cell degranulation and release of chemokines that attract immune cells. Mrgprb2-/- mice exhibited reduced brain inflammation after stroke, leading to attenuated infarct size, and reduced mortality. Further, we show evidence that Mrgprb2-/- mice recruited fewer skull bone marrow neutrophils into the brain, suggesting a novel mechanism whereby mast cells regulate skull bone marrow recruitment. We demonstrated that the human ortholog of this receptor, MRGPRX2, is expressed in human meningeal mast cells, and that these cells are activated in stroke patients. Further, substance P, a known ligand of Mrgprb2/X2, is increased in stroke patient serum. Lastly, osthole-treated mice have reduced post-stroke brain inflammation and improved functional outcomes, confirming that pharmacologic inhibition of Mrgprb2 is a promising therapeutic strategy.
Conclusions: Our study identifies Mrgprb2 as a key receptor which triggers mast cell activity in stroke and initiates brain inflammation. Mrgprb2/X2 provides a specific and druggable target to attenuate post-stroke inflammation and holds potential to meaningfully alter the clinical course for stroke patients.
Methods: We performed MCA occlusion (MCAO) on wild-type and Mrgprb2-null (Mrgprb2-/-) mice and assessed stroke volume, and behavior. We used flow cytometry and ELISA for immune cell and cytokine profiling to evaluate inflammation. Human stroke patient dura and blood was sampled to assess mast cell activity and to identify potential ligands for Mrgprb2 activation. Lastly, we used osthole, a Mrgprb2 inhibitor, as a post-MCAO treatment to determine if pharmacologic inhibition can attenuate post-stroke deficits in mice.
Results: We found that Mrgprb2 is activated in meningeal mast cells after stroke, causing mast cell degranulation and release of chemokines that attract immune cells. Mrgprb2-/- mice exhibited reduced brain inflammation after stroke, leading to attenuated infarct size, and reduced mortality. Further, we show evidence that Mrgprb2-/- mice recruited fewer skull bone marrow neutrophils into the brain, suggesting a novel mechanism whereby mast cells regulate skull bone marrow recruitment. We demonstrated that the human ortholog of this receptor, MRGPRX2, is expressed in human meningeal mast cells, and that these cells are activated in stroke patients. Further, substance P, a known ligand of Mrgprb2/X2, is increased in stroke patient serum. Lastly, osthole-treated mice have reduced post-stroke brain inflammation and improved functional outcomes, confirming that pharmacologic inhibition of Mrgprb2 is a promising therapeutic strategy.
Conclusions: Our study identifies Mrgprb2 as a key receptor which triggers mast cell activity in stroke and initiates brain inflammation. Mrgprb2/X2 provides a specific and druggable target to attenuate post-stroke inflammation and holds potential to meaningfully alter the clinical course for stroke patients.
More abstracts on this topic:
84 Immune checkpoint profiling in major aortic diseases leads to identification of potential roles of CD155-CD206 pathway in suppressing inflammation and immune responses
Shao Ying, Saaoud Fatma, Xu Keman, Lu Yifan, Jiang Xiaohua, Wang Hong, Yang Xiaofeng
Aging Heart Failure with Preserved Ejection Fraction is Mediated by Noncoding RNAsChakraborty Sankalpa, Dickerson Bryce, Bounds Curren, Lemus Sophia, Hickman Caleb, Rajagopalan Viswanathan
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)