Final ID: 103
Anticoagulation versus Antiplatelets Across Subgroups of Embolic Stroke of Undetermined Source: A Meta-Analysis of Seven Randomized Controlled Trials
Abstract Body: Background:
The term embolic stroke of undetermined source (ESUS) encompasses a substantial but heterogeneous population of patients with ischemic stroke, underscoring the importance of identifying personalized treatment strategies. In subgroups of patients randomized in ESUS trials, we evaluated the effectiveness of anticoagulation compared to antiplatelet therapy in secondary ischemic stroke prevention.
Methods:
A study-level meta-analysis was conducted of randomized controlled trials of patients with ESUS, comparing anticoagulation to antiplatelet therapy. The primary efficacy outcome was recurrent ischemic stroke and safety outcomes were major bleeding and death. Subgroups assessed were age, sex, presence of patent foramen ovale (PFO), left atrial enlargement (LAE), and atrial cardiopathy. Pooled relative risks (RRs) were meta-analyzed. Cochrane Risk of Bias Tool 2.0 (RoB 2) was used for risk of bias assessment.
Results:
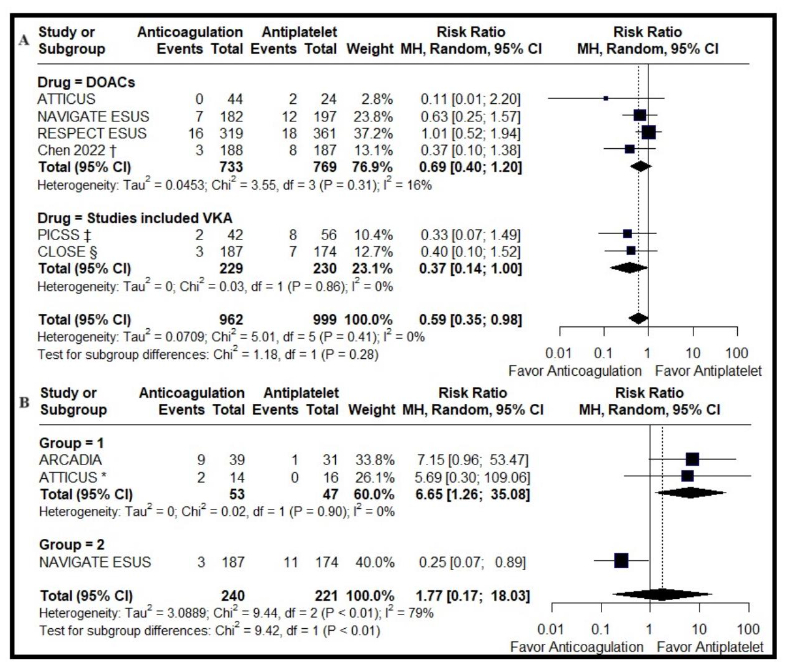
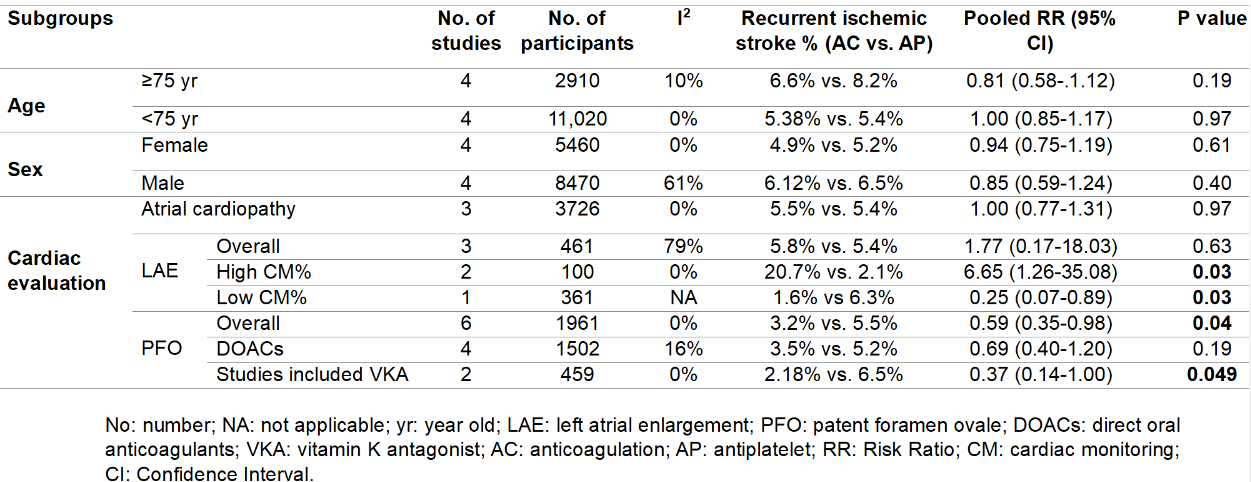
A total of seven randomized controlled trials involving 14,804 patients were analyzed, with 7,406 patients treated with anticoagulation, and 7,398 treated with antiplatelet therapy. Compared with antiplatelet therapy, anticoagulation was associated with a similar rate of recurrent ischemic stroke (RR 0.91, 95% CI 0.80-1.05; I2 = 0%). In ESUS with PFO, anticoagulation was associated with significantly lower risk of ischemic stroke (RR 0.59, 95% CI 0.35-0.98; I2 = 0%). Heterogeneity was present in those with LAE: antiplatelet therapy was superior in trials allowing cardiac monitoring after randomization (RR 6.65, 95% CI 1.26-35.08; I2 = 0%), but anticoagulation was superior in trials prohibiting cardiac monitoring after randomization (RR 0.25 95% CI 0.07-0.89). Subgroups based on age, sex, or presence of atrial cardiopathy did not benefit from anticoagulation over antiplatelet therapy.
Conclusions:
In this meta-analysis, an empiric anticoagulation approach is not beneficial for patients with ESUS. This finding highlights the importance of an individualized treatment strategy. Such a strategy should include prolonged cardiac monitoring for atrial fibrillation, particularly in patients with moderate to severe LAE. Anticoagulation treatment showed promise in patients with medically treated PFO. Other subgroups did not benefit from anticoagulation therapy. Large prospective studies within ESUS subgroups are needed to validate our findings.
The term embolic stroke of undetermined source (ESUS) encompasses a substantial but heterogeneous population of patients with ischemic stroke, underscoring the importance of identifying personalized treatment strategies. In subgroups of patients randomized in ESUS trials, we evaluated the effectiveness of anticoagulation compared to antiplatelet therapy in secondary ischemic stroke prevention.
Methods:
A study-level meta-analysis was conducted of randomized controlled trials of patients with ESUS, comparing anticoagulation to antiplatelet therapy. The primary efficacy outcome was recurrent ischemic stroke and safety outcomes were major bleeding and death. Subgroups assessed were age, sex, presence of patent foramen ovale (PFO), left atrial enlargement (LAE), and atrial cardiopathy. Pooled relative risks (RRs) were meta-analyzed. Cochrane Risk of Bias Tool 2.0 (RoB 2) was used for risk of bias assessment.
Results:
A total of seven randomized controlled trials involving 14,804 patients were analyzed, with 7,406 patients treated with anticoagulation, and 7,398 treated with antiplatelet therapy. Compared with antiplatelet therapy, anticoagulation was associated with a similar rate of recurrent ischemic stroke (RR 0.91, 95% CI 0.80-1.05; I2 = 0%). In ESUS with PFO, anticoagulation was associated with significantly lower risk of ischemic stroke (RR 0.59, 95% CI 0.35-0.98; I2 = 0%). Heterogeneity was present in those with LAE: antiplatelet therapy was superior in trials allowing cardiac monitoring after randomization (RR 6.65, 95% CI 1.26-35.08; I2 = 0%), but anticoagulation was superior in trials prohibiting cardiac monitoring after randomization (RR 0.25 95% CI 0.07-0.89). Subgroups based on age, sex, or presence of atrial cardiopathy did not benefit from anticoagulation over antiplatelet therapy.
Conclusions:
In this meta-analysis, an empiric anticoagulation approach is not beneficial for patients with ESUS. This finding highlights the importance of an individualized treatment strategy. Such a strategy should include prolonged cardiac monitoring for atrial fibrillation, particularly in patients with moderate to severe LAE. Anticoagulation treatment showed promise in patients with medically treated PFO. Other subgroups did not benefit from anticoagulation therapy. Large prospective studies within ESUS subgroups are needed to validate our findings.
More abstracts on this topic:
3-HKA Promotes the Vascular Remodeling after Stroke by Modulating the Activation of A1/A2 Reactive Astrocytes
Chen Jun-min, Shi Guang, Yu Lulu, Shan Wei, Zhang Xiangjian, Wang Qun
A Multi-Center Clinic Site Comparison of Patient-level factors Affecting Oral Anticoagulation Prescription for Atrial FibrillationIqbal Fatima, Hoang Kenneth, Chiadika Simbo
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)