Final ID: WMP111
Progranulin Enhances the Engraftment of Transplanted Human induced Pluripotent Stem Cell-derived Cerebral Neurons in Cell Replacement Therapy
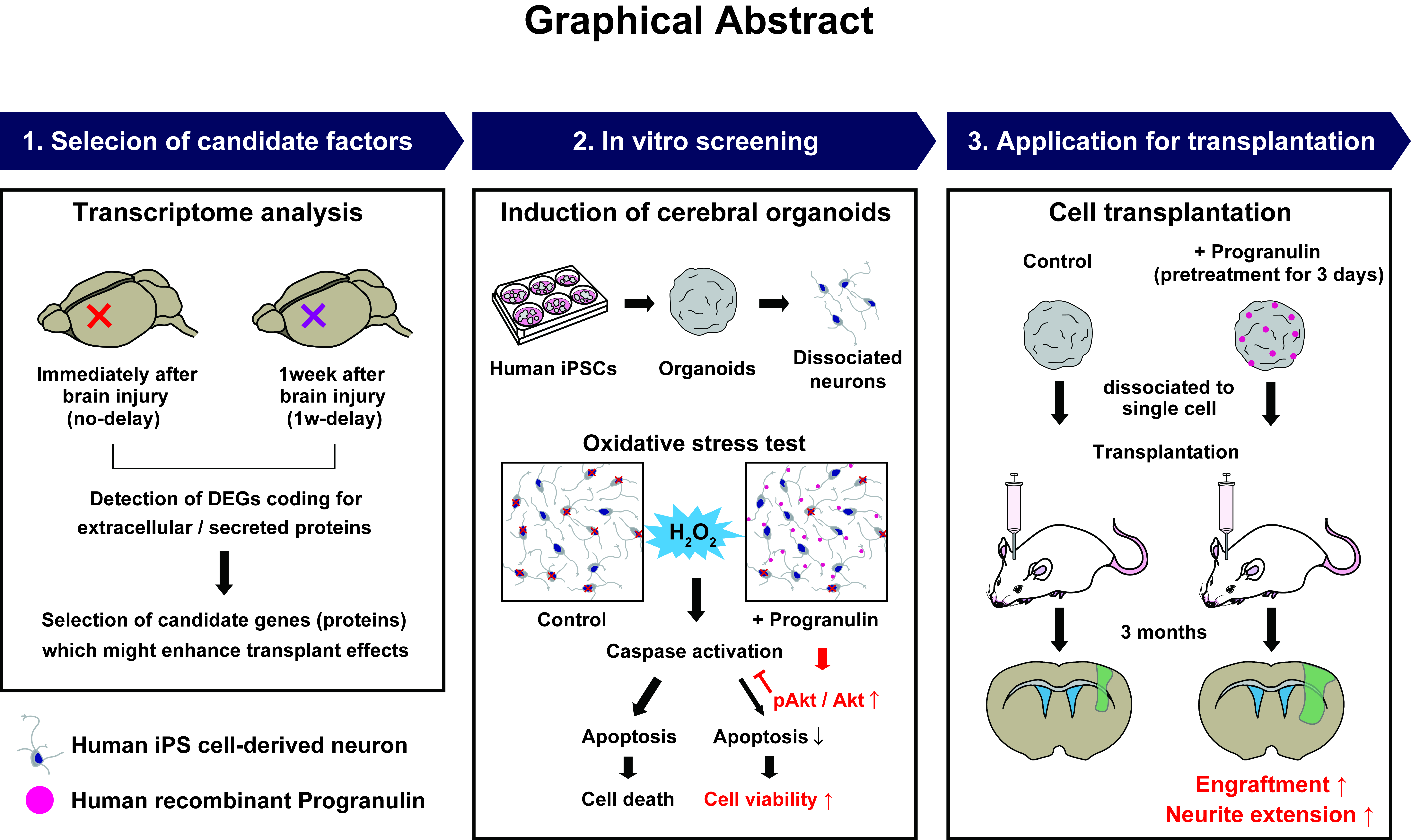
Abstract Body: Background: Stroke or traumatic brain injury (TBI), due to damage to the cerebral motor cortex, often results in significant motor dysfunction and disabilities. Cell replacement therapy emerges as a prospective alternative treatment, promising to restore the impaired neural circuits and facilitate functional recovery. The current study aimed to systemically identify new factors capable of enhancing the efficacy of cell transplantation with human-induced pluripotent stem cell-derived cerebral organoids (hiPSC-COs). Earlier research demonstrated the effectiveness of delaying the transplantation procedure by 1 week and we hypothesized in this study that brain tissues 1 week after brain damage possess a more favorable environment for cell transplantation when compared to immediately after injury.
Methods and Results: Using rodent models, we made a transcriptomic comparison to differentiate gene expression between these two temporal states in order to discern novel factors that could potentiate the therapeutic impact of cell transplantation. Ultimately, 7 candidate genes coding for secreted and extracellular proteins (apolipoprotein D, cathepsin D, cathepsin S, lysozyme 2, secreted phosphoprotein 1, granulin, and secreted protein acidic and cysteine rich) were selected through the transcriptome analysis. In controlled in vitro conditions, recombinant human progranulin (rhPGRN) bolstered the survival rate of dissociated neurons sourced from hiPSC-COs by approximately 25% under oxidative stress. Further experiments revealed that this increase in viability was attributable to a reduction in apoptosis via Akt phosphorylation. In addition, rhPGRN pretreatment before in vivo transplantation experiments augmented the number of engrafted cells about 3.3 times compared to the control group and facilitated neurite elongation along the host brain’s corticospinal tracts in rodent models. Subsequent histological assessments at 3 months post-transplantation revealed an elevated presence of graft-derived subcerebral projection neurons—crucial elements for reconstituting neural circuits—in the rhPGRN-treated group.
Conclusion: Our data highlight the potential of PGRN as a neurotrophic factor suitable for incorporation into hiPSC-CO-based cell therapies for stroke or TBI.
Methods and Results: Using rodent models, we made a transcriptomic comparison to differentiate gene expression between these two temporal states in order to discern novel factors that could potentiate the therapeutic impact of cell transplantation. Ultimately, 7 candidate genes coding for secreted and extracellular proteins (apolipoprotein D, cathepsin D, cathepsin S, lysozyme 2, secreted phosphoprotein 1, granulin, and secreted protein acidic and cysteine rich) were selected through the transcriptome analysis. In controlled in vitro conditions, recombinant human progranulin (rhPGRN) bolstered the survival rate of dissociated neurons sourced from hiPSC-COs by approximately 25% under oxidative stress. Further experiments revealed that this increase in viability was attributable to a reduction in apoptosis via Akt phosphorylation. In addition, rhPGRN pretreatment before in vivo transplantation experiments augmented the number of engrafted cells about 3.3 times compared to the control group and facilitated neurite elongation along the host brain’s corticospinal tracts in rodent models. Subsequent histological assessments at 3 months post-transplantation revealed an elevated presence of graft-derived subcerebral projection neurons—crucial elements for reconstituting neural circuits—in the rhPGRN-treated group.
Conclusion: Our data highlight the potential of PGRN as a neurotrophic factor suitable for incorporation into hiPSC-CO-based cell therapies for stroke or TBI.
More abstracts on this topic:
Beyond Repair: Resurrecting Neurons through Stem Cell Therapy
Hamdan Tesnim, Witt Iryna, Ascandar Nameer, Basaran Ali, Brewer Yukiko A., Chyshkevych Iryna
Eicosapentaenoic Acid (EPA) Increases Expression of Nrf2-mediated Antioxidant Response Element and Heme Oxygenase-1 in Cytokine-Activated Endothelial CellsSherratt Samuel, Libby Peter, Dunbar Richard, Bhatt Deepak, Mason Preston
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.
Rate this abstract
(Maximum characters: 500)