Final ID: 8
Modified Rankin Score at 90 Days vs. NIH Stroke Scale at 24 Hours as Primary Outcome in Acute Stroke Trials
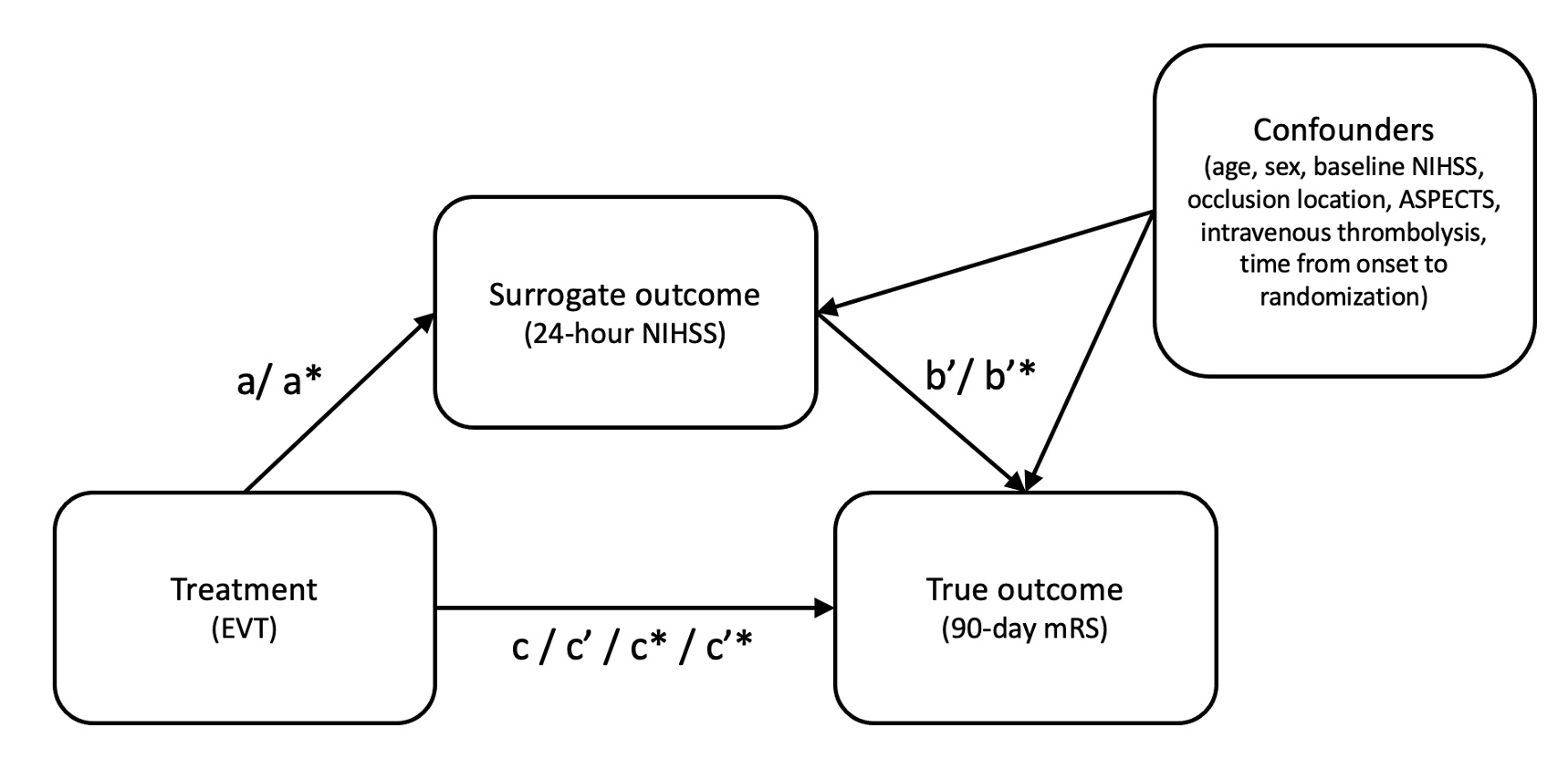
Methods: Post-hoc analysis from the HERMES collaboration that pooled data from 7 randomized controlled EVT trials. Validity of 24-hour NIHSS as a surrogate outcome for 90-day mRS was assessed in a causal mediation model (Figure 1). A 7-point ordinal NIHSS score was generated by grouping 24-hour NIHSS, including death as a separate category (“ordinal” NIHSS). EVT effect sizes and sample sizes required for detecting EVT benefit with 80% power were compared when using granular 24-hour NIHSS, ordinal 24-hour NIHSS, 90-day mRS, and hierarchical outcome (win-ratio) that combines 90-day mRS and 24-hour NIHSS. Subgroup analyses were performed in patients with baseline NIHSS<10 and ≥25.
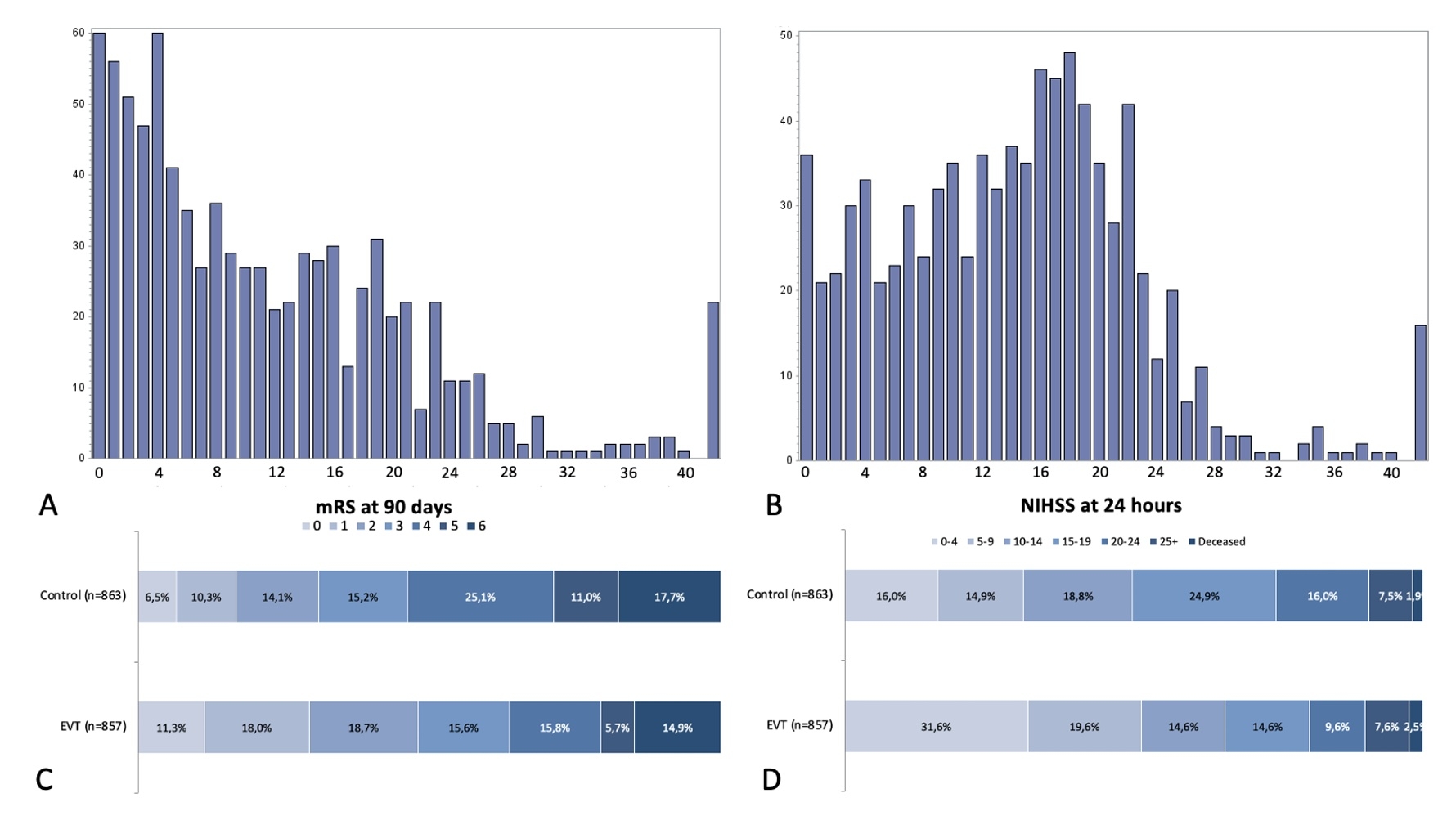
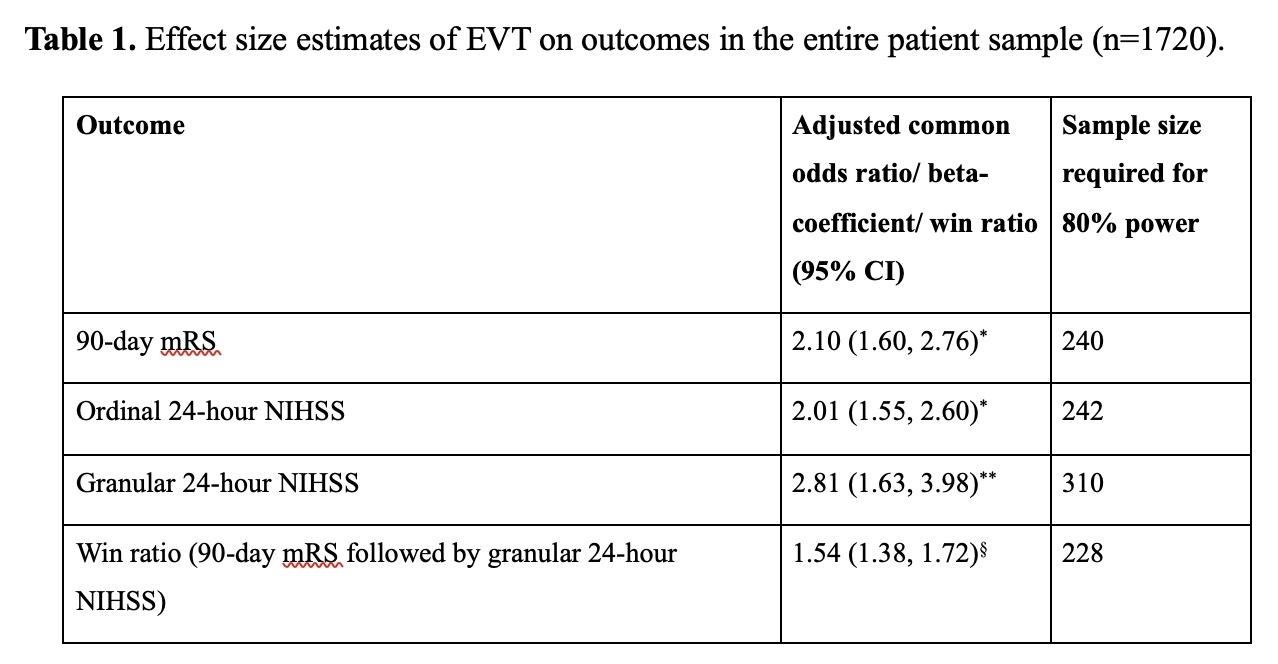
Results: A total of 1720 patients were included. Median 90-day mRS in the EVT/control arms was 3.0(IQR:1.0-4.0)/4.0(IQR:2.0-5.0) and median 24-hour NIHSS was 9.0(IQR:3.0-17.0)/14.0(IQR:7.5-19.0), see Figure 2. 24-hour NIHSS mediated the association between EVT and 90-day mRS and met the criteria for a surrogate outcome. Effect sizes were highest and sample sizes required to detect EVT benefit smallest for the win ratio approach (228), followed by 90-day mRS(240) and ordinal 24-hour NIHSS(242), see Table 1. In subgroup analyses of patients with baseline NIHSS<10 and≥25, ordinal 24-hour NIHSS resulted in the highest effect size/ smallest sample size.
Conclusion: 24-hour NIHSS may be a valid surrogate outcome for 90-day mRS in acute ischemic stroke patients undergoing EVT, with a similar EVT effect size compared to 90-day mRS. It could potentially enhance detection of EVT benefit in patient subgroups with very low or very high baseline NIHSS. Combining 90-day mRS and 24-hour NIHSS in an ordered hierarchical could improve detection of EVT treatment effect compared to 90-day mRS.
- Ospel, Johanna ( University of Calgary , Calgary , Alberta , Canada )
- Dippel, Diederik ( ERASMUS MC , Bunnik , Netherlands )
- Majoie, Charles ( AMC , Amsterdam , Netherlands )
- Jovin, Tudor ( Cooper Neurological Institute , Haddonfield , New Jersey , United States )
- Campbell, Bruce ( Royal Melbourne Hospital , Parkville , Victoria , Australia )
- Mitchell, Peter ( The Royal Melbourne Hospital , Parkville , Victoria , Australia )
- Bracard, Serge ( Nancy University , Nancy , France )
- Guillemin, Francis ( Nancy University , Nancy , France )
- Hill, Michael ( UNIVERSITY CALGARY , Calgary , Alberta , Canada )
- Goyal, Mayank ( university of calgary , Calgary , Alberta , Canada )
- Brown, Scott ( BRIGHT Research Partners , Mooresville , North Carolina , United States )
- Bosshart, Salome ( University of Calgary , Calgary , Alberta , Canada )
- Stebner, Alexander ( University of Calgary , Calgary , Alberta , Canada )
- Uchida, Kazutaka ( Hyogo Medical University , Nishinomiya , Japan )
- Demchuk, Andrew ( University of Calgary , Calgary , Alberta , Canada )
- Saver, Jeffrey ( GEFFEN SCHOOL OF MEDICINE AT UCLA , Los Angeles , California , United States )
- White, Phil ( Newcastle University , Newcastle upon Tyne , United Kingdom )
- Muir, Keith ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
Meeting Info:
Session Info:
More abstracts on this topic:
Ang Kevin Royce, Juangco Dan, Hernandez Maria Kim
High Mechanical Thrombectomy Procedural Volume is Not a Reliable Predictor ofImproved Thrombectomy Outcomes in Patients with Acute Ischemic Stroke in the
United States
Qureshi Adnan, Spiotta Alejandro, Zaidi Syed, Kwok Chun Shing, Maqsood Hamza, Bhatti Ibrahim, Ford Daniel, Gomez Camilo, Hanley Daniel, Hassan Ameer, Nguyen Thanh, Siddiq Farhan
More abstracts from these authors:
Tariq Muhammad Bilal, Jovin Tudor, Mitchell Peter, Campbell Bruce, Bracard Serge, Guillemin Francis, Muir Keith, White Phil, Dippel Diederik, Hill Michael, Goyal Mayank, Brown Scott, Saver Jeffrey, Liebeskind David, Tiedt Steffen, Lun Ronda, Albers Gregory, Mun Katherine, Majoie Charles, Demchuk Andrew
Association between Time and Severe Hypoperfusion with Risk of Hemorrhagic Transformation in Thrombectomy Stroke PatientsPensato Umberto, Goyal Mayank, Demchuk Andrew, Hill Michael, Ospel Johanna
Readers' Comments
We encourage you to enter the discussion by posting your comments and questions below.
Presenters will be notified of your post so that they can respond as appropriate.
This discussion platform is provided to foster engagement, and simulate conversation and knowledge sharing.
You have to be authorized to post a comment. Please, Login or Signup.