Final ID: FR614
Hepatic Autophagy Deficiency Increases ANGPTL4 Biosynthesis And Reduces Vascular Contractility Through Integrin Signaling in Spontaneously Hypertensive BPH/2J Mice
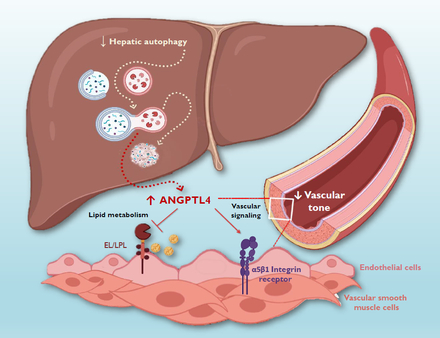
Abstract Body: Autophagy is essential for maintaining cellular homeostasis, and its disruption in hypertension has been linked to vascular dysfunction. We previously reported that angiopoietin-like protein 4 (ANGPTL4), a lipid metabolism hepatokine, is elevated in autophagy-deficient mice, and BPH/2J hypertensive mice have diminished hepatic autophagy and elevated circulating ANGPTL4. Classically, ANGPTL4 inhibits lipoprotein lipase and endothelial lipase thereby eliciting its hypertriglyceridemic effects. ANGPTL4 has also been shown to activate α5β1 integrin receptors. However, its role in regulating vascular tone during hypertension remains unclear. We hypothesized that the increased ANGPTL4 seen in hypertensive BPH/2J mice would mediate hypercontractility. To test this hypothesis, we performed wire myography on mesenteric resistance arteries (MRAs) in naive C57BL/6J mice, as well as in BPH/2J (hypertensive) and BPN/3J (normotensive) mice chronically treated with a GalNAc-siRNA targeting ANGPTL4 (2 mg/kg). The GalNAc conjugation targets the siRNA specifically to the liver via sialic acid receptor, which has a high affinity for liver cells and rapid internalization. Pharmacological interventions in naive mice included incubations with recombinant ANGPTL4 (rANGPTL4; 2 µg/mL) and a selective α5β1 integrin agonist (HY-139702; 10 µM for 30 min). Opposing our original hypothesis, we observed that incubation with rANGPTL4 significantly reduced maximal phenylephrine-induced contraction (Emax: 6.00 ± 0.29 vs. 7.95 ± 0.44 mN/mm; p = 0.0009). Likewise, α5β1 integrin activation decreased contraction at 1 µM phenylephrine (5.11 ± 0.51 vs. 7.95 ± 0.44 mN/mm; p= 0.0017), suggesting that the anti-contractile effect of ANGPTL4 may be mediated through the α5β1 integrin receptor. Supporting these acute data, we observed that ANGPTL4 silencing in hypertensive BPH/2J mice enhanced mesenteric resistance vasoconstriction to phenylephrine (6.62 ± 0.74 vs. 2.82 ± 0.74 mN/mm; p= 0.0058). Importantly, restoration of ANGPTL4 with rANGPTL4 ex vivo significantly attenuated phenylephrine-induced contraction in MRAs from ANGPTL4-silenced mice (4.28 ± 0.74 vs. 8.48 ± 0.74 mN/mm; p= 0.0002), but had no significant effect in MRAs from control siRNA-treated mice. Collectively, these findings indicate that hepatic autophagy deficiency increases ANGPTL4 bioavailability in hypertension, where it possibly plays a compensatory vasomodulatory role by attenuating excessive vascular tone via integrin signaling.
More abstracts on this topic:
Association Between Elevated Lipoprotein(a) and New-Onset Atrial Fibrillation: A Retrospective Analysis Using the TriNetX Research Network
Qadeer Abdul, Akbar Usman, Ahmed Faizan, Shabbir Muhammad Raffey, Aamir Muhammad, Fouad Michele, Khan Allahdad, Khawar Muneeb, Pathak Prutha, Hassan Furqan, Hotwani Priya, Khan Sardar Muhammad Imran, Shafique Nouman
Age-Related Impairment of Mitochondrial Protein Turnover Exacerbates Pathogenesis of Heart Failure with Preserved Ejection Fraction in Old MiceKobak Kamil, Zarzycka Weronika, King Catherine, Borowik Agnieszka, Peelor Frederick, Kinter Michael, Miller Benjamin, Chiao Ying Ann