Final ID: TAC207
Hypertension-Driven Bone Loss and Immune Activation in an Aging Mice: Pharmacologic Prevention
Abstract Body: Background: Hypertension (HTN) is linked to immune activation and bone marrow myelopoiesis, processes that drive osteoclastogenesis and bone loss. Myelopoiesis and osteoclast formation are dependent on colony-stimulating factor 1 (CSF1) while intermittent parathyroid hormone 1-34 (PTH) prevents age-related osteoporosis.
Purpose: We sought to determine if HTN induces bone loss in young and aged mice and examined therapeutic interventions targeting both bone formation using intermittent PTH and bone resorption using a CSF1 antibody (CSF1i).
Methods: 48 male C57BL/6J mice were divided into two groups: 4 months (young) and 16 months (aged), and were subdivided to receive Angiotensin II (Ang II, 490 ng/kg/day X 6 weeks) or vehicle via subcutaneous osmotic pumps. 50 male aged mice, comprising 10 controls and 40 receiving Ang-II, the later, divided into four groups treated with vehicle, PTH, CSF1i, or PTH+CSF1i for the last 4 weeks of Ang II infusion. Plasma, bone marrow, femurs, and vertebrae were analyzed using ELISA, flow cytometry, micro-computed tomography, and 3-point bending.
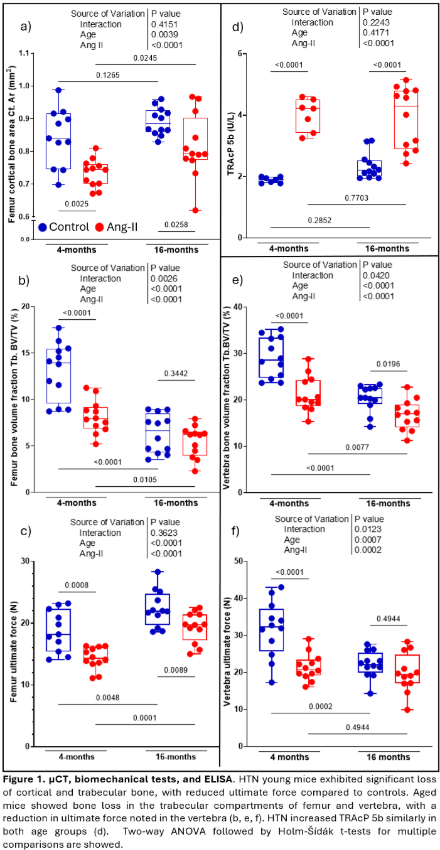
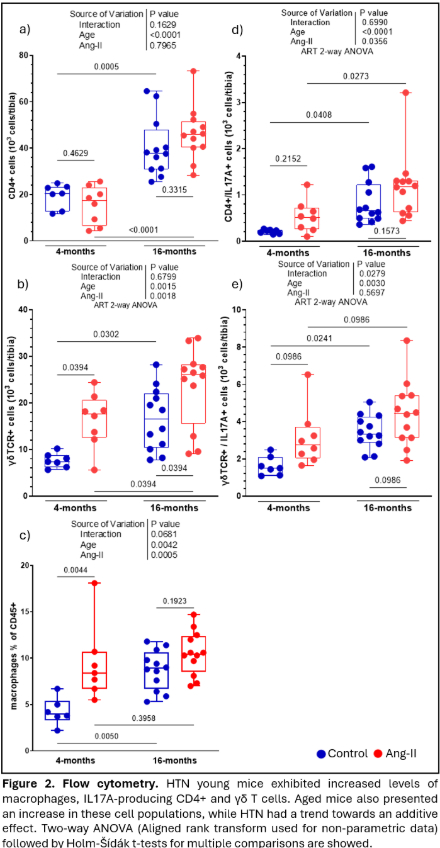
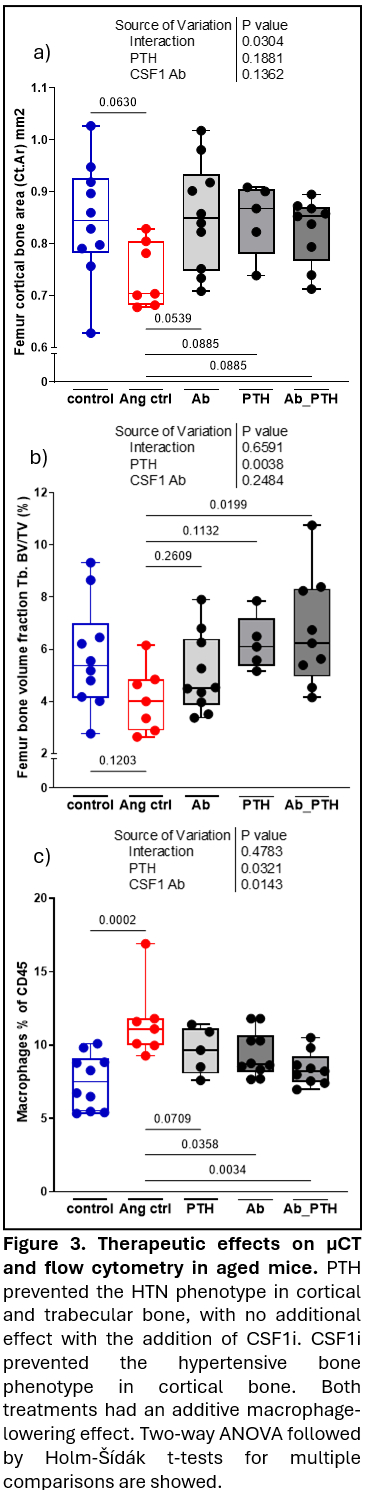
Results: In young mice, HTN decreased trabecular bone, weakened bone strength, and increased bone marrow macrophages (precursors to osteoclasts), and IL-17A producing CD4+ and γδ T cells. In trabecular bone of the lumbar vertebra and distal femoral metaphysis, aging caused bone loss that was equivalent to HTN in young mice. In aged mice, HTN minimally further reduced trabecular bone properties yet modestly affected cortical bone. Like HTN in young mice, aging alone increased macrophages, CD4+ T cells, and γδ T cells producing IL-17A, with little change with HTN. HTN increased circulating TRAcP 5b (marker of bone resorption) in both young and old mice. PTH prevented the hypertensive bone phenotype in cortical and trabecular compartments, without additional benefits when CSF1i was added. CSF1i prevented the hypertensive bone phenotype in cortical bone only. Both treatments had an additive macrophage-lowering effect. Neither PTH or CSF1i affected IL-17A+ T-cells.
Conclusion: HTN mimics aging in bone health, driven by myelopoiesis and T cell-mediated inflammation. Therapeutic interventions with PTH and CSF1i mitigate macrophage expansion. CSF1i protects cortical bone from HTN-induced deterioration, while PTH benefited both cortical and trabecular bone. These findings underscore distinct yet complementary therapeutic roles for PTH and CSF1i in hypertensive bone disease in aging.
Purpose: We sought to determine if HTN induces bone loss in young and aged mice and examined therapeutic interventions targeting both bone formation using intermittent PTH and bone resorption using a CSF1 antibody (CSF1i).
Methods: 48 male C57BL/6J mice were divided into two groups: 4 months (young) and 16 months (aged), and were subdivided to receive Angiotensin II (Ang II, 490 ng/kg/day X 6 weeks) or vehicle via subcutaneous osmotic pumps. 50 male aged mice, comprising 10 controls and 40 receiving Ang-II, the later, divided into four groups treated with vehicle, PTH, CSF1i, or PTH+CSF1i for the last 4 weeks of Ang II infusion. Plasma, bone marrow, femurs, and vertebrae were analyzed using ELISA, flow cytometry, micro-computed tomography, and 3-point bending.
Results: In young mice, HTN decreased trabecular bone, weakened bone strength, and increased bone marrow macrophages (precursors to osteoclasts), and IL-17A producing CD4+ and γδ T cells. In trabecular bone of the lumbar vertebra and distal femoral metaphysis, aging caused bone loss that was equivalent to HTN in young mice. In aged mice, HTN minimally further reduced trabecular bone properties yet modestly affected cortical bone. Like HTN in young mice, aging alone increased macrophages, CD4+ T cells, and γδ T cells producing IL-17A, with little change with HTN. HTN increased circulating TRAcP 5b (marker of bone resorption) in both young and old mice. PTH prevented the hypertensive bone phenotype in cortical and trabecular compartments, without additional benefits when CSF1i was added. CSF1i prevented the hypertensive bone phenotype in cortical bone only. Both treatments had an additive macrophage-lowering effect. Neither PTH or CSF1i affected IL-17A+ T-cells.
Conclusion: HTN mimics aging in bone health, driven by myelopoiesis and T cell-mediated inflammation. Therapeutic interventions with PTH and CSF1i mitigate macrophage expansion. CSF1i protects cortical bone from HTN-induced deterioration, while PTH benefited both cortical and trabecular bone. These findings underscore distinct yet complementary therapeutic roles for PTH and CSF1i in hypertensive bone disease in aging.
More abstracts on this topic:
A Case of Right Coronary Artery Chronic Total Occlusion in a Transplanted Heart: To Stent or Not to Stent?
A Longitudinal 20-year Analysis Indicates Acceleration of Cardiometabolic Comorbidities on Dementia Risk
Krayem Hussein, Cooke Richard, Abouzaki Nayef, Kutkut Issa
A Longitudinal 20-year Analysis Indicates Acceleration of Cardiometabolic Comorbidities on Dementia Risk
Lihua Huang, Danish Muhammad, Auyeung Tw, Jenny Lee, Kwok Timothy, Abrigo Jill, Wei Yingying, Lo Cecilia, Fung Erik