Final ID: 61
Protective role of caspase-1 inhibition in experimental model of neonatal hyperoxia-induced cardiopulmonary dysfunction in adults born preterm.
Abstract Body: Background: Adults born preterm are at an increased risk of systemic hypertension and cardiopulmonary morbidities. However, little is known of the underlying mechanisms connecting neonatal hyperoxia (O2) exposure to these long-term cardiovascular consequences in adults born preterm. Pro-inflammatory caspase-1 mediates the activation of cytokines IL-1β, and pore-forming protein Gasdermin-D.
Objective: Test the hypothesis that caspase-1 inhibition prevents long-term cardiopulmonary consequences of preterm birth.
Design/Methods: Newborn mice (n=60) exposed to normoxia (RA) or hyperoxia (O2) from postnatal day (P) 1 to 14 were randomly assigned to receive daily intraperitoneal (IP) injections of caspase-1 inhibitor (VX-765, 50 ug/Kg) or DMSO as placebo. The mouse pups recovered in RA into adulthood until 3 months of age. The effect of caspase-1 inhibition on Blood pressure (BP), exercise endurance, cardiac function, systemic vascular stiffness, pulmonary vascular remodeling was assessed at 3 months.
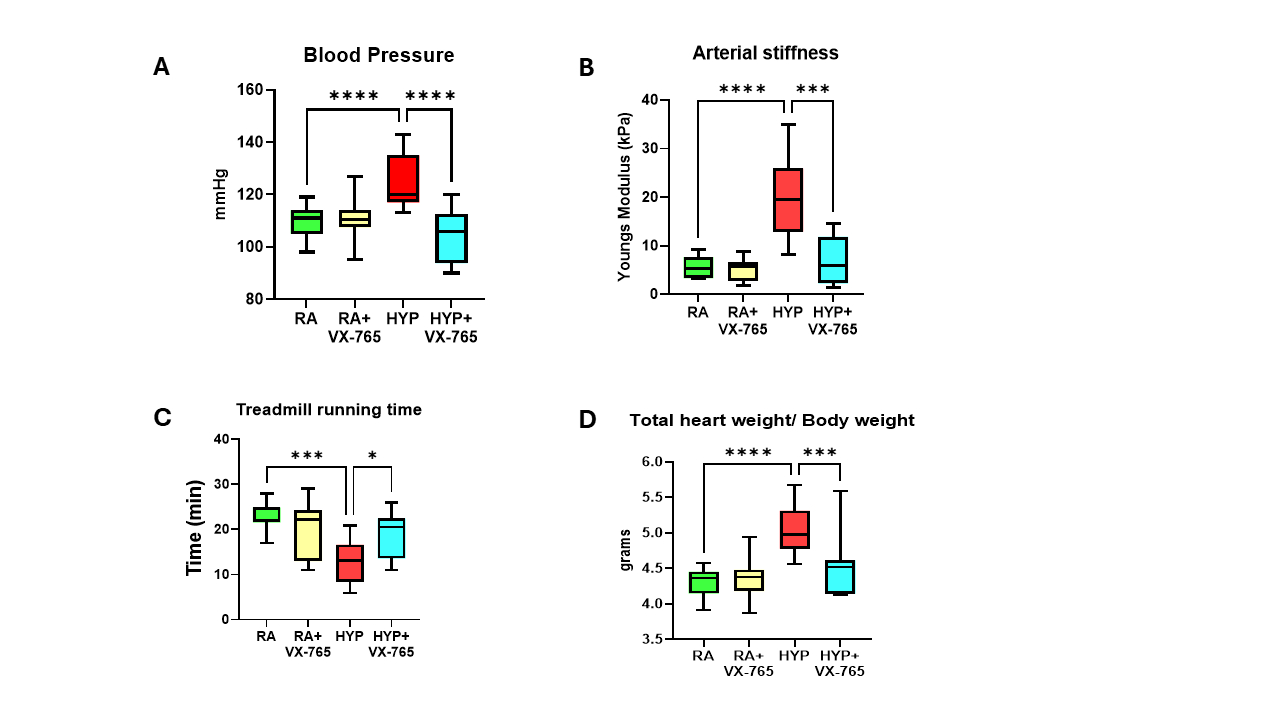
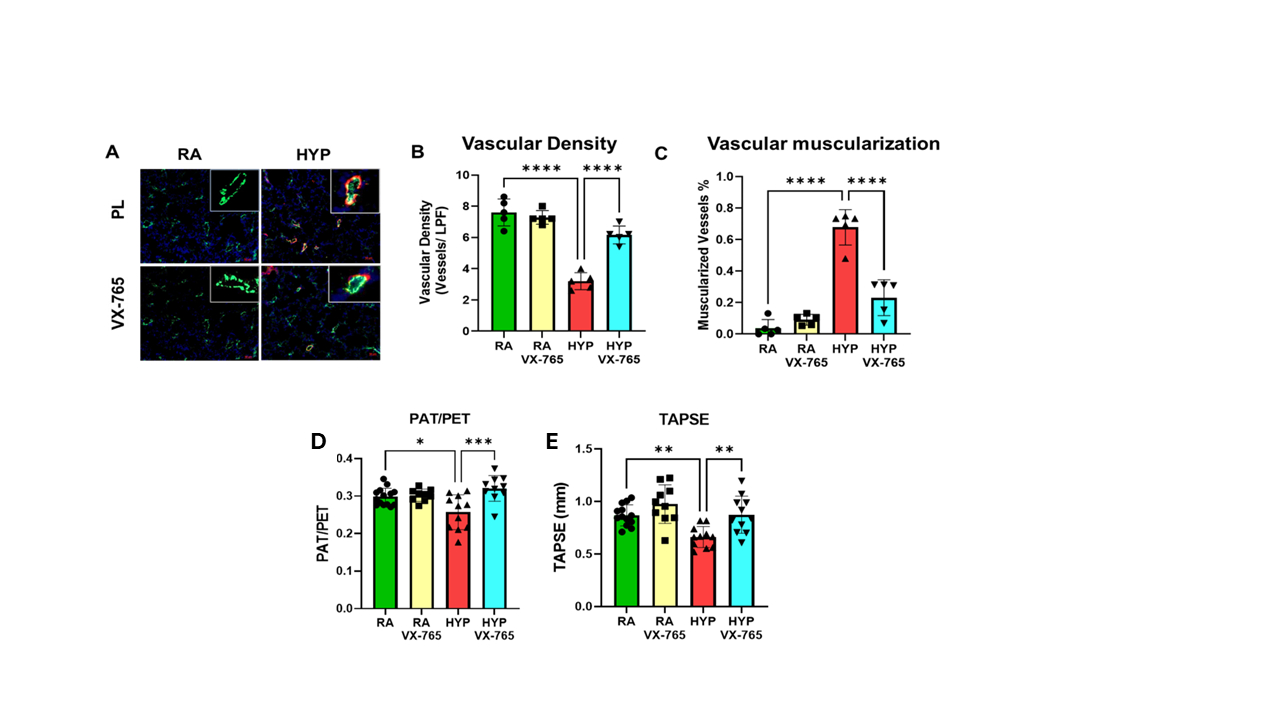
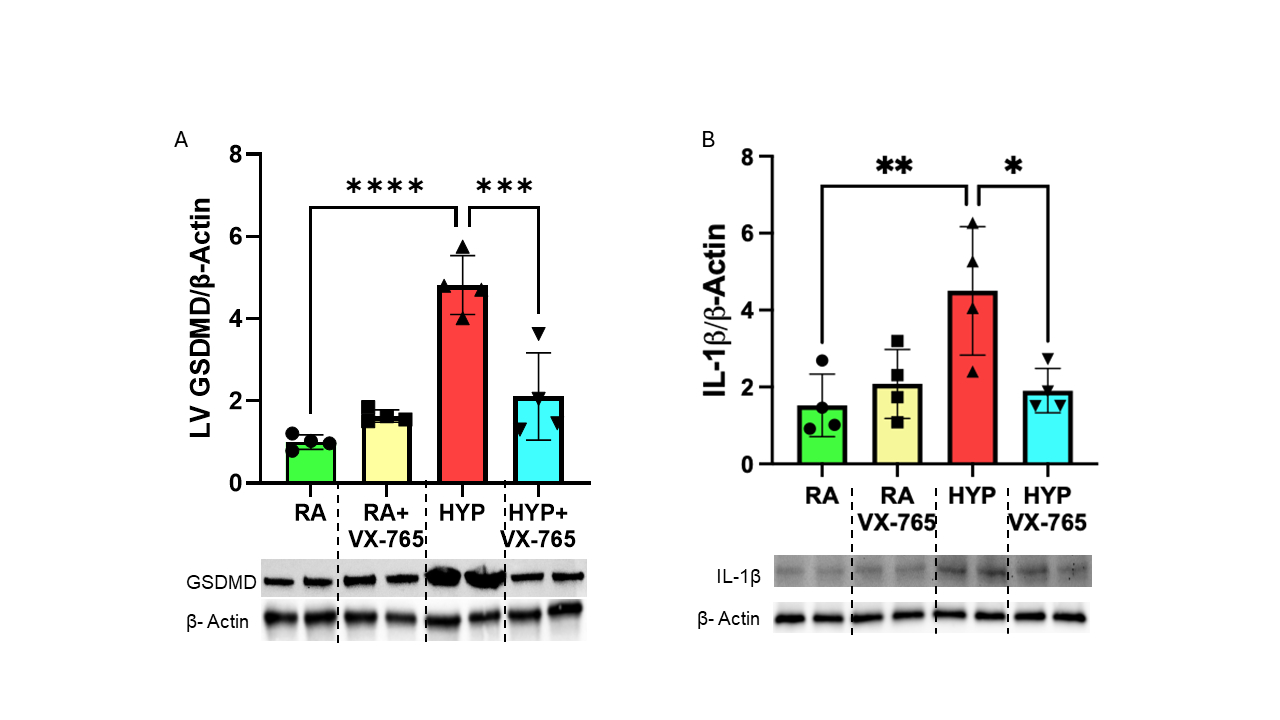
Results: Adult rats exposed to neonatal O2 had significantly increased BP associated with vascular stiffness, reduced exercise capacity, cardiac hypertrophy, and cardiovascular inflammation. Caspase-1 inhibition prevented systemic hypertension, systemic vascular stiffness, improved exercise tolerance and decreased cardiac hypertrophy (Fig 1A-D) in adult mice exposed to neonatal O2. Moreover, caspase -1 inhibition improved pulmonary vascular remodeling and pulmonary hypertension in these mice (Fig 2A-E). Additionally, western blot analysis demonstrated that early supplementation of VX-765 in O2 exposed mice led to reduced cardiac proinflammatory markers GSDMD and IL-1β in adulthood (Fig 3A-B).
Conclusion: Neonatal O2 exposure predisposes to cardiopulmonary morbidities in adults born preterm. Inhibition of caspase-1 reduces cardiovascular inflammation and cardiopulmonary dysfunction in mice exposed to hyperoxia during the neonatal period. The functional and molecular impact of the use of caspase-1 suggests that it can be used as a novel preventive strategy for long-term cardiopulmonary dysfunction due to neonatal O2 exposure in preterm survivors.
Objective: Test the hypothesis that caspase-1 inhibition prevents long-term cardiopulmonary consequences of preterm birth.
Design/Methods: Newborn mice (n=60) exposed to normoxia (RA) or hyperoxia (O2) from postnatal day (P) 1 to 14 were randomly assigned to receive daily intraperitoneal (IP) injections of caspase-1 inhibitor (VX-765, 50 ug/Kg) or DMSO as placebo. The mouse pups recovered in RA into adulthood until 3 months of age. The effect of caspase-1 inhibition on Blood pressure (BP), exercise endurance, cardiac function, systemic vascular stiffness, pulmonary vascular remodeling was assessed at 3 months.
Results: Adult rats exposed to neonatal O2 had significantly increased BP associated with vascular stiffness, reduced exercise capacity, cardiac hypertrophy, and cardiovascular inflammation. Caspase-1 inhibition prevented systemic hypertension, systemic vascular stiffness, improved exercise tolerance and decreased cardiac hypertrophy (Fig 1A-D) in adult mice exposed to neonatal O2. Moreover, caspase -1 inhibition improved pulmonary vascular remodeling and pulmonary hypertension in these mice (Fig 2A-E). Additionally, western blot analysis demonstrated that early supplementation of VX-765 in O2 exposed mice led to reduced cardiac proinflammatory markers GSDMD and IL-1β in adulthood (Fig 3A-B).
Conclusion: Neonatal O2 exposure predisposes to cardiopulmonary morbidities in adults born preterm. Inhibition of caspase-1 reduces cardiovascular inflammation and cardiopulmonary dysfunction in mice exposed to hyperoxia during the neonatal period. The functional and molecular impact of the use of caspase-1 suggests that it can be used as a novel preventive strategy for long-term cardiopulmonary dysfunction due to neonatal O2 exposure in preterm survivors.
More abstracts on this topic:
Conditional deletion of ADAM17 (A disintegrin and metalloprotease 17) in microglia attenuates hypertension and associated inflammation.
Mohan Uma Priya, Dinh Paula, Yue Xinping, Lazartigues Eric
A Retrospective Analysis of Measured Versus Estimated Resting Oxygen Uptake for Derivation of Fick Cardiac Output in Patients in the Cardiac Intensive Care UnitMinasian Alexandra, Dudzinski David, Shelton Ken, Malhotra Rajeev, Lewis Gregory, Mcginnis Shaina, Rouvina Jennifer, Newlands Chloe, Griskowitz Catharine, Kowal Alyssa, Kelly Katherine, Oloughlin Joseph, Wallace Dayquan