Final ID: Or116
One Carbon Metabolism Defect In Failing Hearts
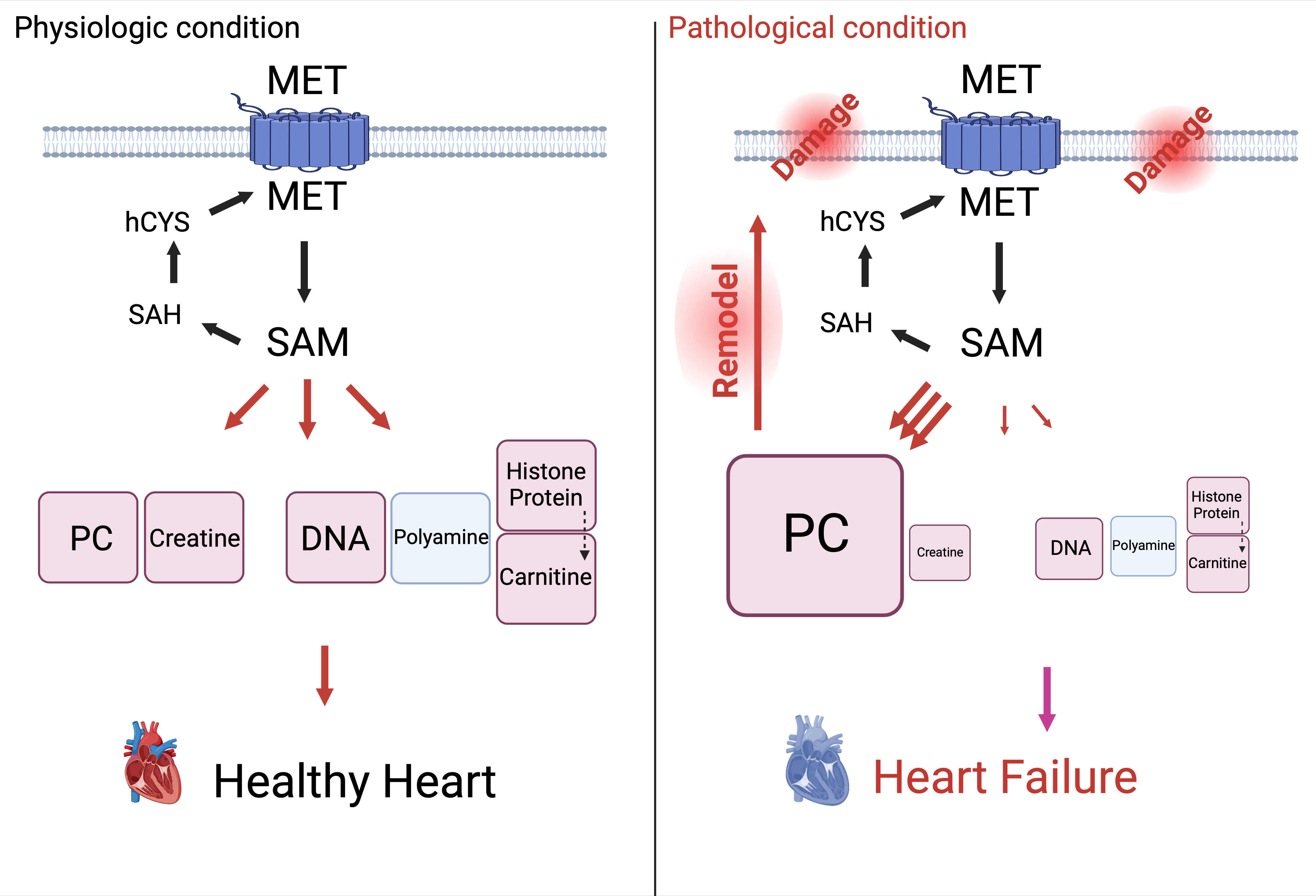
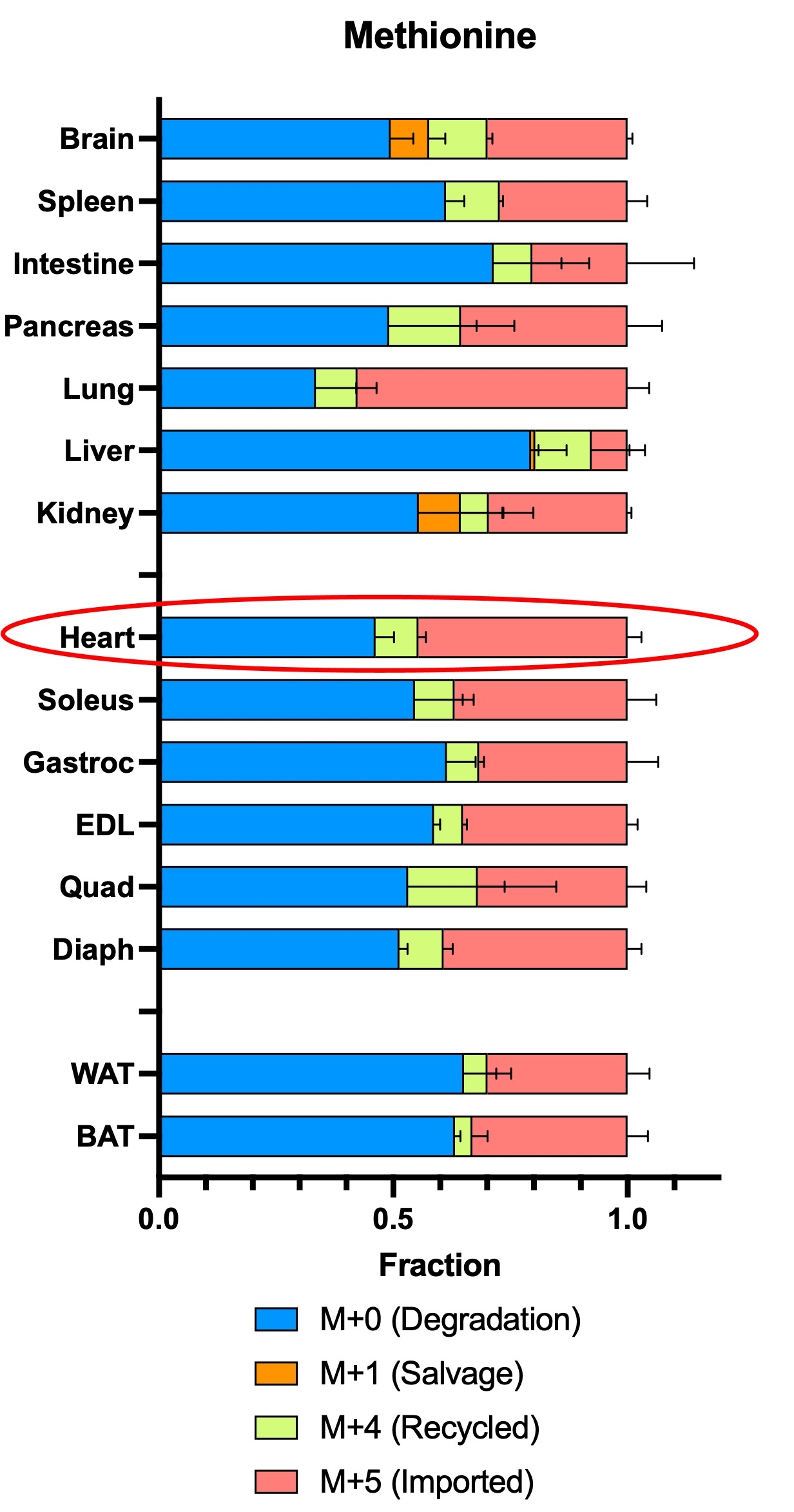
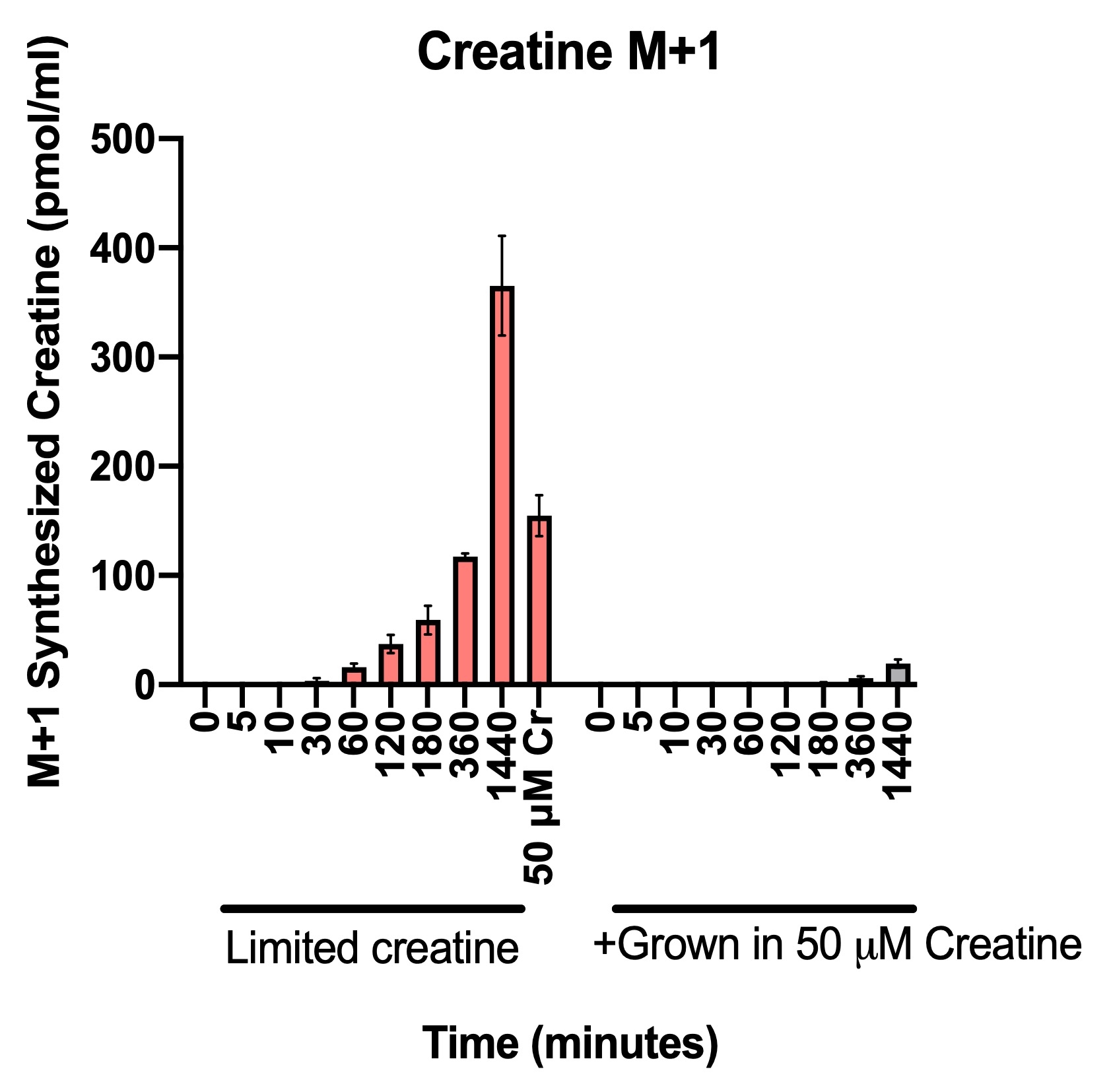
Abstract Body: Aberrant cardiac metabolism has long been hypothesized to contribute to heart failure (HF). However, understanding of cardiac metabolism in HF remains incomplete, especially in humans. To fill this gap, we performed metabolomic and RNA sequencing analysis of 48 non-failing (NF) and 39 dilated cardiomyopathy (DCM) hearts. Among many differences, metabolites from one-carbon metabolism were significantly reduced, especially in methionine cycle; methionine (Met) and S-adenosylmethionine (SAM), a universal methyl donor of methylation. We next studied the mechanism leading to decreased Met/SAM in DCM hearts. We first quantitatively defined methionine cycle in the heart: studies in neonatal rat ventricular myocytes (NRVMs), and in vivo steady-state infusions with isotope tracing, showed that Met was mostly imported or degraded from protein rather than recycled from homocysteine (~10%), suggesting that a defect in recycling is unlikely to explain low Met/SAM in DCM hearts. We next explored the impact of low SAM on cardiac function by generating mice with cardiac specific deletion of SAM synthase. Constitutive KO mice were largely embryonic lethal, and the few survivors showed dramatic systolic dysfunction and died within 12 weeks. Inducing loss of SAM synthase in adults led to 50% mortality after 2 weeks, again with marked cardiac dysfunction. Finally, we explored potential mechanisms by which low SAM can drive heart failure. In human DCM, numerous methylation products were low, including most notably creatine, a SAM methylation product and essential metabolite for cardiac energetics. Creatine is not thought to be synthesized in the heart, but we found that, to our surprise, NRVMs synthesized ~50% of their creatine pool when external supply was low, and synthesis was dependent on SAM availability. Moreover, in vivo steady state infusions revealed that in intact animals, even in the absence of stressors, ~10% of creatine in the heart is synthesized locally. In summary, we find that: (1) the majority of Met in cardiomyocyte is imported or derived from protein degradation, with rapid turnover; (2) Met and its product SAM are reduced in human DCM; (3) loss of SAM in murine heart leads to profound heart failure; (4) creatine is surprisingly synthesized from SAM in cardiomyocytes, and its levels are reduced in human DCM. Together, these findings suggest that a defective Met cycle may contribute to low creatine and ultimately HF.
More abstracts on this topic:
A Hypertrophic Cardiomyopathy-Based Model Estimate of the Prevalence of Danon Disease in the United States
Maron Martin, Massera Daniele, Manganaro Susan, Bailey Miranda, Rehbein Fletcher, Taylor Matthew
A human cardiomyocyte model of CD36 haploinsufficiency uncovers fatty acid oxidation deficits driving dilated cardiomyopathyAl Sayed Zeina, Klattenhoff Carla, Aragam Krishna, Ellinor Patrick, Willcox Jon, Zheng Alice, Koledova Vera, Srivastava Salil, Yin Xiaofei, Chaffin Mark, Rigaud Vagner, Kovacs-bogdan Erika