Final ID: Tu099
GDF11 accelerates NF-kB-mediated inflammation prior to reduction in cardiac fibrosis in response to experimental pressure overload.
Abstract Body: Background: Cardiac fibrosis is a common pathophysiology in a broad variety of heart diseases. GDF11, a member of the TGF-beta superfamily, can reduce cardiac hypertrophy and fibrosis in the pressure overload model of experimental Trans Aortic Constriction (TAC). However, the molecular mechanisms by which GDF11 can reduce cardiac fibrosis are incompletely described.
Hypothesis: GDF11 is known to activate both NF-kB as well as SMAD2/3 signaling which are essential in cardiac fibrosis and hypertrophy processes. We hypothesized that exogenous GDF11 can change the dynamics of early inflammatory NF-kB target genes during the response to pressure overload.
Methods: We used mice implanted with AngII-osmotic pumps to induce left ventricular fibrosis. The mice were harvested at different time points: 3, 7, 14, and 28 days post-pump implantation (ppi) to follow the evolution of cardiac fibrosis and cardiac hypertrophy, and to identify the role of GDF11 in modulating these processes.
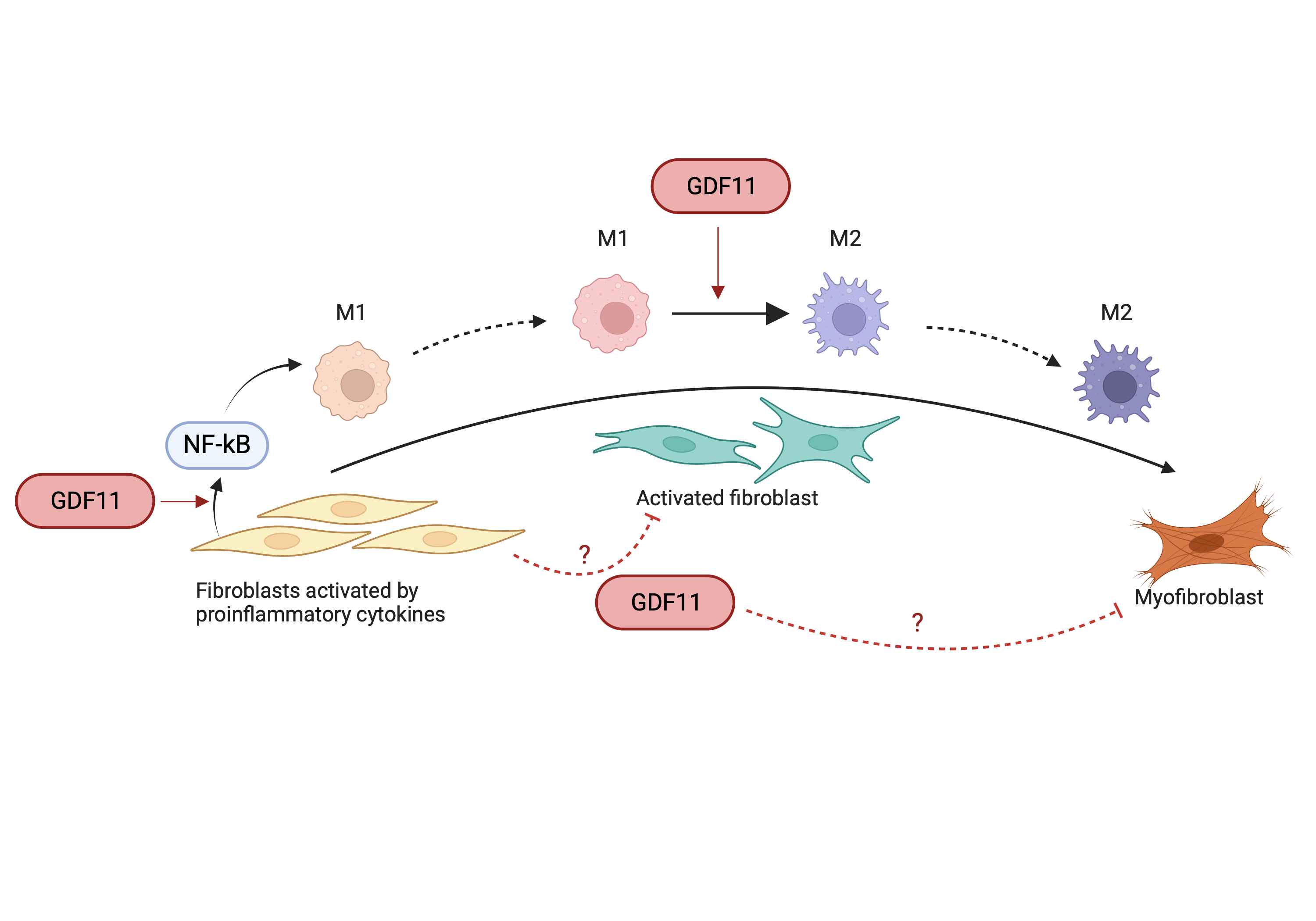
Results: GDF11 delivery (1mg/kg every other day) in AngII mice (AngII+GDF11) induced earlier expression of NF-kB target genes (IL-1beta, IL-6, TNF-alpha – p>0.05) compared to AngII+Veh control group at 3d ppi, revealing accelerated activation of NF-kB response pathways in AngII+GDF11 group. This is associated with faster recruitment of the M1 macrophage population observed by an increased expression of CD86 or iNOS at 3d ppi. We also observed decreased markers of fibroblast activation after GDF11 supplementation, with less collagen deposition (F-CHP) and fibrosis (Masson Trichrome) at 28 days.
Conclusions: Our data suggest that exogenous GDF11 changes the dynamics of NF-kB inflammatory mechanisms while reducing later fibrosis. This new finding could suggest that the dynamics of the inflammatory response early after pressure overload is a critical factor in cardiac fibrosis.
Hypothesis: GDF11 is known to activate both NF-kB as well as SMAD2/3 signaling which are essential in cardiac fibrosis and hypertrophy processes. We hypothesized that exogenous GDF11 can change the dynamics of early inflammatory NF-kB target genes during the response to pressure overload.
Methods: We used mice implanted with AngII-osmotic pumps to induce left ventricular fibrosis. The mice were harvested at different time points: 3, 7, 14, and 28 days post-pump implantation (ppi) to follow the evolution of cardiac fibrosis and cardiac hypertrophy, and to identify the role of GDF11 in modulating these processes.
Results: GDF11 delivery (1mg/kg every other day) in AngII mice (AngII+GDF11) induced earlier expression of NF-kB target genes (IL-1beta, IL-6, TNF-alpha – p>0.05) compared to AngII+Veh control group at 3d ppi, revealing accelerated activation of NF-kB response pathways in AngII+GDF11 group. This is associated with faster recruitment of the M1 macrophage population observed by an increased expression of CD86 or iNOS at 3d ppi. We also observed decreased markers of fibroblast activation after GDF11 supplementation, with less collagen deposition (F-CHP) and fibrosis (Masson Trichrome) at 28 days.
Conclusions: Our data suggest that exogenous GDF11 changes the dynamics of NF-kB inflammatory mechanisms while reducing later fibrosis. This new finding could suggest that the dynamics of the inflammatory response early after pressure overload is a critical factor in cardiac fibrosis.
More abstracts on this topic:
A Cellular Mechanism Mediating Lipomatous Metaplasia In the Infarcted Heart.
Tuleta Izabela, Frangogiannis Nikolaos, Venugopal Harikrishnan, Huang Shuaibo, Humeres Claudio, Hernandez Velasco Silvia, Hanna Anis, Kubota Akihiko, O'leary Kevin, Zheng Deyou
A Mast Cell-Specific Receptor Mediates Post-Stroke Brain Inflammation Via a Dural-Brain AxisKothari Ruchita, Caplan Justin, Gonzalez L. Fernando, Jackson Christopher, Bettegowda Chetan, Huang Judy, Koehler Raymond, Tamargo Rafael, Xu Risheng, Dong Xinzhong, Abdulrahim Mostafa, Oh Hyun Jong, Capuzzi Daniel, Nair Sumil, Zhang Yaowu, Limjunyawong Nathachit, Saini Sarbjit, Kim Jennifer