Final ID: We023
P53 Activation Promotes Maturational Characteristics of Pluripotent Stem Cell-derived Cardiomyocytes in 3D Suspension Culture via FOXO-FOXM1 Regulation
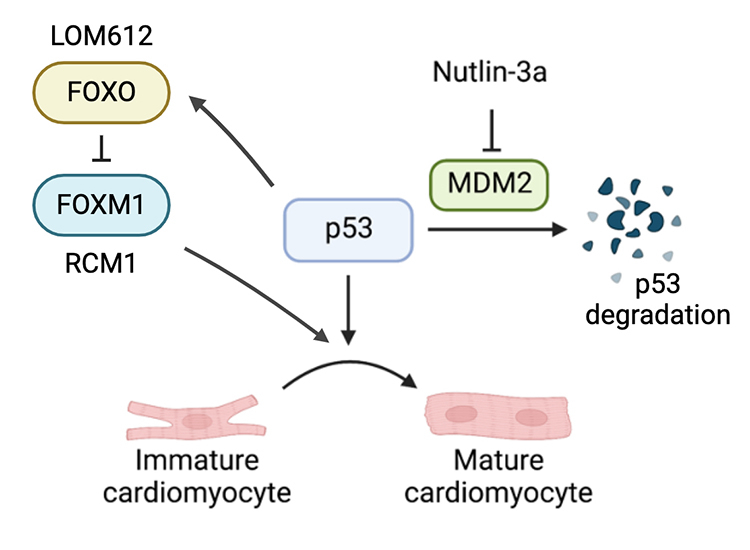
Hypothesis: The forkhead box (FOX) family of transcription factors can regulate maturation in neonatal cardiomyocytes through a balance between FOXO and FOXM1. We also previously found that p53 activation could enhance hiPSC-CM maturation. Therefore, we hypothesized that p53 activation increases FOXO and decreases FOXM1 to promote hiPSC-CM maturation in three-dimensional (3D) suspension culture.
Methodology: 3D cultures of hiPSC-CMs were treated with Nutlin-3a (p53 activator), LOM612 (FOXO activator), AS1842856 (FOXO inhibitor), or RCM-1 (FOXM1 inhibitor), starting 2 days after onset of beating. The hiPSC-CMs were assessed for maturation in metabolic, contractile, and electrophysiological properties, by Seahorse mito stress test, Multi electrode array, and VALA kinetic image cytometer respectively.
Results: P53 activation promoted FOXO upregulation and FOXM1 downregulation in hiPSC-CMs, measured by RT-qPCR and immunostaining. Alongside this, p53 activation also promoted hiPSC-CM metabolic and contractile maturational characteristics, seen by increase in oxygen consumption and beat amplitude respectively. FOXO inhibition significantly decreased expression of cardiac-specific markers such as TNNT2 and eliminated spontaneous beating. In contrast, FOXO activation or FOXM1 inhibition promoted maturational characteristics of hiPSC-CMs such as increase in contractility, oxygen consumption, and voltage peak maximum upstroke velocity. Further, by single-cell RNAseq, FOXO activated groups showed increase in cardiac maturational pathways compared against DMSO control groups.
Conclusions: These results show that p53 activation promotes FOXO and suppresses FOXM1, which modulate hiPSC-CM maturation in 3D suspension. Our study expands current understanding of hiPSC-CM maturational mechanisms in a clinically-relevant 3D culture system.
- Velayutham, Nivedhitha ( Harvard University , Cambridge , Massachusetts , United States )
- Shaw, Jeanna ( Harvard University , Cambridge , Massachusetts , United States )
- Bouffard, Aldric ( Harvard University , Cambridge , Massachusetts , United States )
- Morgan, Sokol ( Harvard University , Cambridge , Massachusetts , United States )
- Mancheno Juncosa, Estela ( Harvard University , Cambridge , Massachusetts , United States )
- Rhoades, Seth ( Bitome, Inc. , Boston , Massachusetts , United States )
- Van Den Berg, Daphne ( Harvard University , Cambridge , Massachusetts , United States )
- Kreymerman, Alexander ( Harvard University , Cambridge , Massachusetts , United States )
- Aoyama, Junya ( Harvard University , Cambridge , Massachusetts , United States )
- Hoefflin, Jens ( Bitome, Inc. , Boston , Massachusetts , United States )
- Ryan, Herb ( Bitome, Inc. , Boston , Massachusetts , United States )
- Garbern, Jessica ( Harvard University , Cambridge , Massachusetts , United States )
- Ho Sui, Shannan ( Harvard School of Public Health , Boston , Massachusetts , United States )
- Lee, Richard ( Harvard University , Cambridge , Massachusetts , United States )
- Elwell, Hannah ( Harvard University , Cambridge , Massachusetts , United States )
- Zhuo, Zhu ( Harvard School of Public Health , Boston , Massachusetts , United States )
- Ruland, Laura ( Harvard University , Cambridge , Massachusetts , United States )
- Elcure Alvarez, Farid ( Harvard University , Cambridge , Massachusetts , United States )
- Frontini, Sara ( Harvard University , Cambridge , Massachusetts , United States )
- Rodriguez Carreras, Yago ( Harvard University , Cambridge , Massachusetts , United States )
- Ricci-blair, Elisabeth ( Harvard University , Cambridge , Massachusetts , United States )
Meeting Info:
Session Info:
Poster Session and Reception 3
Wednesday, 07/24/2024 , 04:30PM - 07:00PM
Poster Session and Reception
More abstracts on this topic:
Wang Haofei, Liu Jiandong, Dong Yanhan, Shi Huitong, Colon Marazzano, Liu Xingyan, Farber Gregory, Qian Yunzhe, Anthony Nicholas, Qian Li
Adducin Promotes Cardiomyocyte Sarcomere DisassemblyXiao Feng, Menendez-montes Ivan, Elhelaly Waleed, Singh Rohit, Sadayappan Sakthivel, Kanchwala Mohammed, Xing Chao, Ladha Feria, Hinson Travis, Hajjar Roger, Hill Joseph, Nguyen Ngoc Uyen Nhi, Sadek Hesham, Wang Ping, Hsu Ching-cheng, Li Shujuan, Thet Suwannee, Kimura Wataru, Luo Xiang, Lam Nicholas
More abstracts from these authors:
Ben Driss Laura, Fandl Hannah, Wifak Imane, Wagner Julian, Kleefeldt Florian, Van Den Berg Daphne, Xu Lucy, Lee Richard
HIV-Nef extracellular vesicles utilize the novel Btk-NFκB-MerTK signaling axis to impair macrophage efferocytosis and promote atherosclerosis: A multiomics studyChelvanambi Sarvesh, Enomoto Takashi, Matamalas Joan, Mukai Shin, Santinelli Pestana Diego, Ge Rile, Perez Katelyn, Kasai Taku, Kuraoka Shiori, Silva Monteiro Gabriel, Tabas Ira, Decano Julius L, Ho Sui Shannan, Sonawane Abhijeet, Singh Sasha, Aikawa Elena, Aikawa Masanori, Nakamura Yuto, Yangihara Yoshihiro, Jule Amelie, Delwarde Constance, Bartoli-leonard Francesca, Jha Prabhash, Lupieri Adrien