Final ID: Tu134
Oncostatin M favors cardiomyocyte survival by promoting glycolysis
Abstract Body: Introduction Cardiomyocytes is in high demand of energy and is susceptible to low oxygen environment. Timely switch of energy metabolism after cardiac ischaemia is helpful for cardiomyocyte preservation. We previously identified a critical role of Oncostatin M (OSM) in cardiomyocyte proliferation. Since the level of OSM quickly rises in myocardium after myocardial injury, OSM may play a protective role in early phase after myocardial injury through metabolic regulation.
Hypothesis We hypothesized that Oncostatin M regulates cardiomyocyte energy metabolism to promote its resistance to hypoxia.
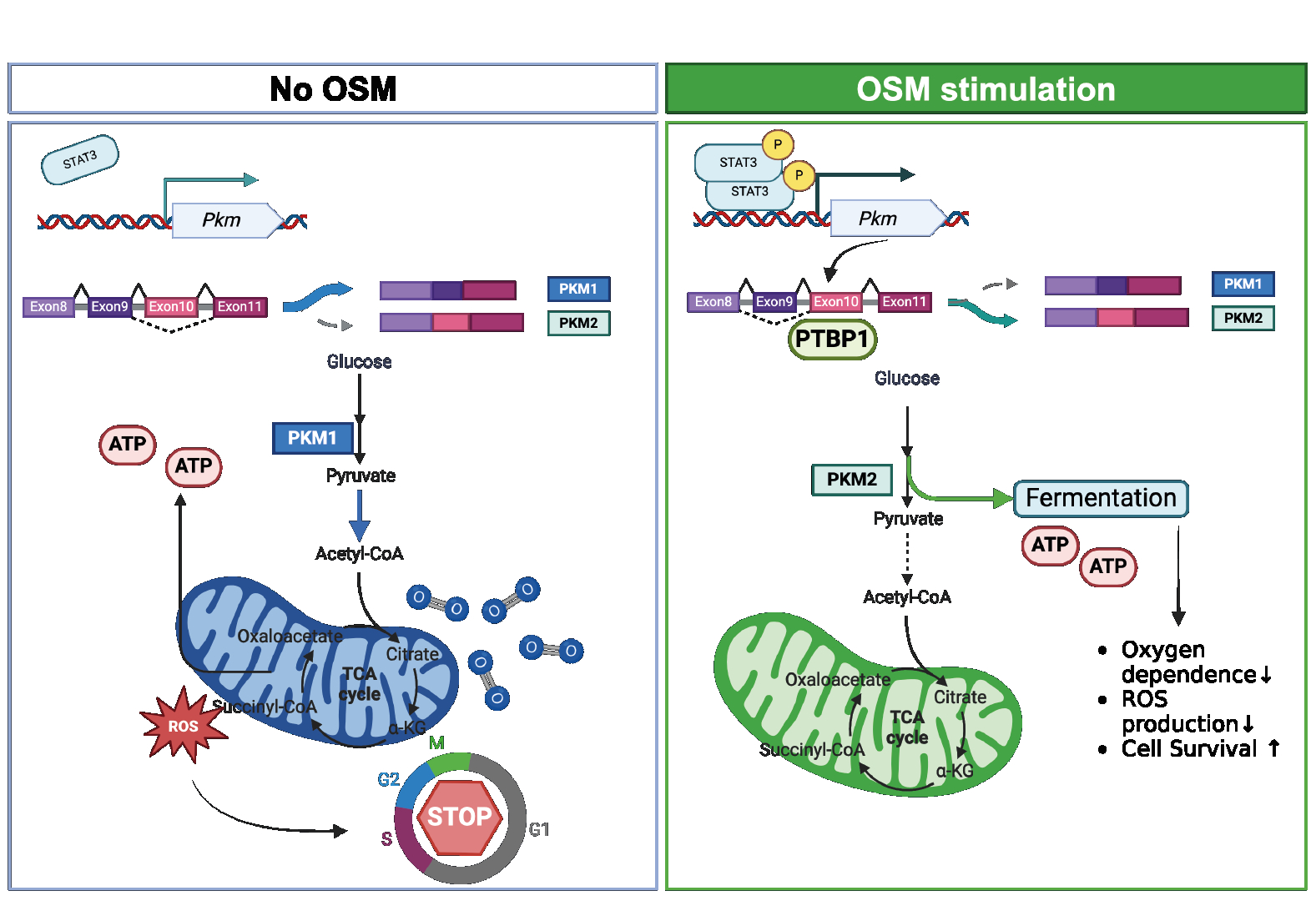
Methods and Results We cultivated cardiomyocytes under hypoxic condition observed that in OSM-treated group, the percentage of TUNEL positive cardiomyocytes significantly decreased compared with that in vehicle-treated group. Published RNA sequencing data from OSM-treated neonatal cardiomyocytes revealed alterations in genes related to energy metabolism. Our Seahorse metabolic stress assays demonstrated that OSM-treated cardiomyocytes exhibited higher glycolytic metabolism and lower mitochondrial respiration than vehicle-treated cells. Pyruvate kinase M2 (PKM2), among the regulatory enzymes, was significantly upregulated in OSM-treated cardiomyocytes as was detected via western-blotting. Interestingly, PKM2 has lower catalytic activity compared to its isotype PKM1, suggesting that the augmented glycolysis might be mediated by PKM2. The mutually exclusive splicing of the gene Pkm between exons 9 and 10 led to the switch between PKM1 and PKM2. qPCR results indicated a 1.5-fold increase in Pkm transcripts and a 2-fold increase in the ratio of Pkm2 to Pkm1, suggesting that OSM concomitantly promoted Pkm transcription as well as alternative splicing of Pkm2. Furthermore, we performed RNA interference to knock down Stat3, the canonical downstream signal of OSM, in cardiomyocytesand observed that OSM failed to upregulate Pkm transcription. ChIP-qPCR analysis on cardiomyocyte cell line HL-1 confirmed an increased STAT3 binding signal upstream of Pkm after OSM treatment. Additionally, RIP-qPCR assays revealed a 3-fold increase in PTBP1-binding signal to Pkm transcripts in cardiomyocytes following OSM treatment.
Conclusions Our results suggest that OSM promotes glycolysis and suppresses mitochondrial respiration in cardiomyocytes by upregulates PKM2 through STAT3 mediated transcription of Pkm and PTBP1-mediated alternative splicing of Pkm transcripts.
Hypothesis We hypothesized that Oncostatin M regulates cardiomyocyte energy metabolism to promote its resistance to hypoxia.
Methods and Results We cultivated cardiomyocytes under hypoxic condition observed that in OSM-treated group, the percentage of TUNEL positive cardiomyocytes significantly decreased compared with that in vehicle-treated group. Published RNA sequencing data from OSM-treated neonatal cardiomyocytes revealed alterations in genes related to energy metabolism. Our Seahorse metabolic stress assays demonstrated that OSM-treated cardiomyocytes exhibited higher glycolytic metabolism and lower mitochondrial respiration than vehicle-treated cells. Pyruvate kinase M2 (PKM2), among the regulatory enzymes, was significantly upregulated in OSM-treated cardiomyocytes as was detected via western-blotting. Interestingly, PKM2 has lower catalytic activity compared to its isotype PKM1, suggesting that the augmented glycolysis might be mediated by PKM2. The mutually exclusive splicing of the gene Pkm between exons 9 and 10 led to the switch between PKM1 and PKM2. qPCR results indicated a 1.5-fold increase in Pkm transcripts and a 2-fold increase in the ratio of Pkm2 to Pkm1, suggesting that OSM concomitantly promoted Pkm transcription as well as alternative splicing of Pkm2. Furthermore, we performed RNA interference to knock down Stat3, the canonical downstream signal of OSM, in cardiomyocytesand observed that OSM failed to upregulate Pkm transcription. ChIP-qPCR analysis on cardiomyocyte cell line HL-1 confirmed an increased STAT3 binding signal upstream of Pkm after OSM treatment. Additionally, RIP-qPCR assays revealed a 3-fold increase in PTBP1-binding signal to Pkm transcripts in cardiomyocytes following OSM treatment.
Conclusions Our results suggest that OSM promotes glycolysis and suppresses mitochondrial respiration in cardiomyocytes by upregulates PKM2 through STAT3 mediated transcription of Pkm and PTBP1-mediated alternative splicing of Pkm transcripts.
More abstracts on this topic:
A Metabolomic Study of Cardiac Dysfunction in Hyperglycemia
Yoshida Yilin, Qi Qibin, Cheng Susan, Kaplan Robert, Rodriguez Carlos, Shah Amil, Yu Bing, Nguyen Ngoc Quynh, Moon Eun Hye, Casey Rebholz, Skali Hicham, Arthur Victoria, Echouffo Justin, Ballantyne Christie, Selvin Elizabeth
84 Immune checkpoint profiling in major aortic diseases leads to identification of potential roles of CD155-CD206 pathway in suppressing inflammation and immune responsesShao Ying, Saaoud Fatma, Xu Keman, Lu Yifan, Jiang Xiaohua, Wang Hong, Yang Xiaofeng