Final ID: Tu039
Intestinal atrophy contributes to low-grade inflammation, cardiac dysfunction and metabolic syndrome-like phenotype in a model of heart failure with preserved ejection fraction.
Abstract Body: Introduction: Heart failure with preserved ejection fraction (HFpEF) comprises half of all HF cases and lacks effective treatment. HFpEF is now seen as a systemic syndrome often linked to comorbidities like hypertension, obesity, and diabetes. These conditions are commonly associated with unhealthy diets rich in fats but lacking in fiber. We explored the effects of a 3-week exposure to excess mineralocorticoids, salt, and high fat diet (HFD) deficient in fiber. We hypothesize that these challenges lead to intestinal atrophy, contributing to low-grade inflammation that promotes the progression of heart failure and metabolic disease.
Methods: We used a mix of 1% saline in drinking water and subcutaneous implantation of controlled-release DOCA pellets (0.83 mg/day) alongside a high-fat diet (60% fat) in 8-week-old female and male C57BL6/J mice. Control mice received a standard chow diet and regular water.
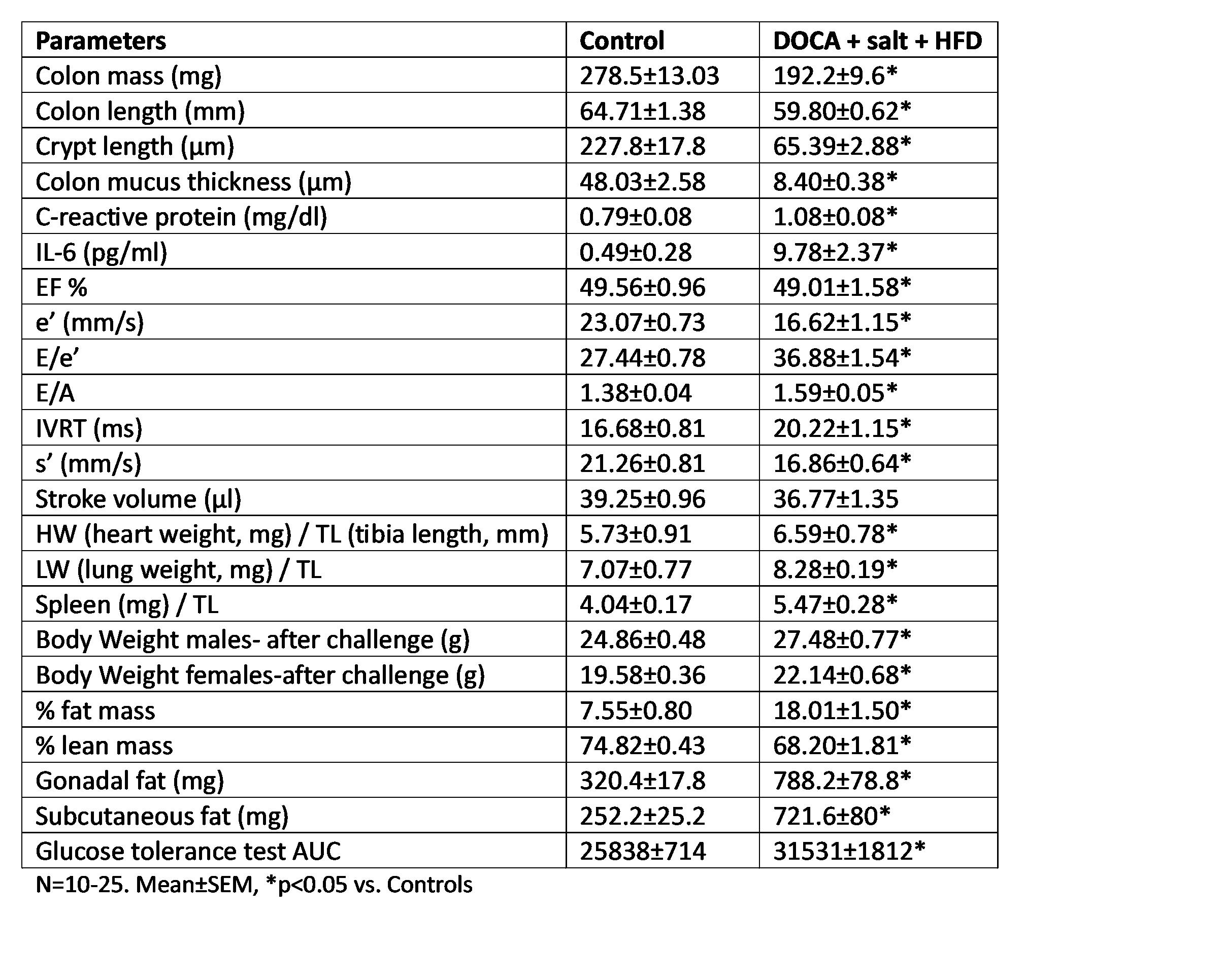
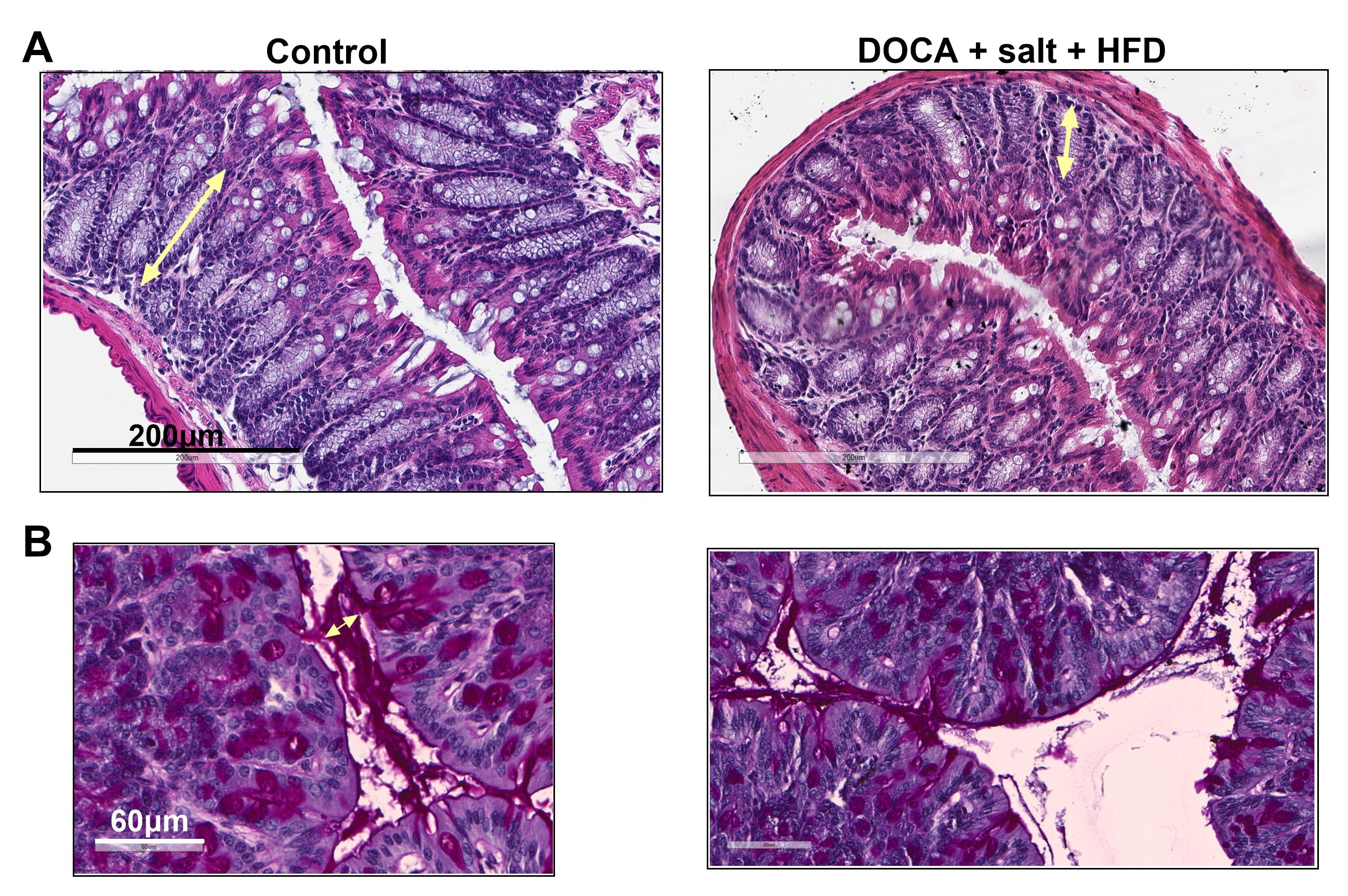
Results: The challenge resulted in significant reductions in colon mass, colon length, crypt length, and mucus layer thickness compared to the control group. Additionally, elevated levels of C-reactive protein and IL-6 were detected in the plasma. These changes were accompanied by alterations in diastolic function, including decreased peak myocardial relaxation velocity (e'), elevated E/e' ratio, increased E/A ratio, and prolonged isovolumetric relaxation time (IVRT), while the ejection fraction (EF) remained unchanged. Contractility parameters, such as peak myocardial contraction velocity (s'), declined in challenged mice, along with diminished stroke volume (SV). We also found increased organ weight in the liver, spleen, and lungs indicative of organ congestion. Furthermore, signs of metabolic syndrome were identified, including increased body weight, increased adiposity characterized by gonadal and subcutaneous fat accumulation, and dysglycemia (see Data Table).
Conclusions: Our data suggests that in this model, heart failure triggers intestinal hypoperfusion, resulting in intestinal atrophy and compromising the integrity of the intestinal lining. Additionally, a diet rich in fat and lacking in fiber alters the gut environment by diminishing mucus production and promoting a closer interaction between the microbiome and host cells. This compromised mucus layer and intestinal barrier facilitates bacterial wall products to move into the circulation, resulting in a pro-inflammatory state that subsequently affects both cardiac function and metabolism.
Methods: We used a mix of 1% saline in drinking water and subcutaneous implantation of controlled-release DOCA pellets (0.83 mg/day) alongside a high-fat diet (60% fat) in 8-week-old female and male C57BL6/J mice. Control mice received a standard chow diet and regular water.
Results: The challenge resulted in significant reductions in colon mass, colon length, crypt length, and mucus layer thickness compared to the control group. Additionally, elevated levels of C-reactive protein and IL-6 were detected in the plasma. These changes were accompanied by alterations in diastolic function, including decreased peak myocardial relaxation velocity (e'), elevated E/e' ratio, increased E/A ratio, and prolonged isovolumetric relaxation time (IVRT), while the ejection fraction (EF) remained unchanged. Contractility parameters, such as peak myocardial contraction velocity (s'), declined in challenged mice, along with diminished stroke volume (SV). We also found increased organ weight in the liver, spleen, and lungs indicative of organ congestion. Furthermore, signs of metabolic syndrome were identified, including increased body weight, increased adiposity characterized by gonadal and subcutaneous fat accumulation, and dysglycemia (see Data Table).
Conclusions: Our data suggests that in this model, heart failure triggers intestinal hypoperfusion, resulting in intestinal atrophy and compromising the integrity of the intestinal lining. Additionally, a diet rich in fat and lacking in fiber alters the gut environment by diminishing mucus production and promoting a closer interaction between the microbiome and host cells. This compromised mucus layer and intestinal barrier facilitates bacterial wall products to move into the circulation, resulting in a pro-inflammatory state that subsequently affects both cardiac function and metabolism.
More abstracts on this topic:
β1 Adrenergic Receptor Autoantibodies Promote Heart Failure Though Activation of Prostaglandin E2 Receptor EP1/Phosphodiesterase 4B Pathway
Cao Ning, Qiu Hui, Li Hongwei
A Case of Steroid-Refractory Immune-checkpoint-inhibitor Induced Myocarditis Responsive to Mycophenolate and Anti-thymocyte globulinDabdoub Jorge, Wilson Michael, Gottbrecht Matthew, Salazar Ryan, Shih Jeffrey