Final ID: We067
A meta-analysis of RNA sequencing data to characterize immune cell activation among patients with cardiac sarcoidosis
Abstract Body:
Introduction
Cardiac sarcoidosis (CS) is a life-threatening auto-immune disorder that causes the formation of
non-caseating granulomas in cardiac tissue. Th17.1 cell have been found to be upregulated in
patients with pulmonary sarcoidosis (PS) although data is lacking among CS patients.
This meta-analysis integrates all publicly available granuloma single-cell and single-nuclei RNA
sequencing data to identify patterns in immune cell activation across different granulomatous
diseases while focusing on CD4+T cell activation among CS patients.
Methods
Five studies were included, spanning cardiac tissue from healthy controls and DCM (Koenig
2022, PMID:35959412), cardiac tissue from ICM and cardiac sarcoidosis (Liu 2022,
PMID:36111531), BAL from beryllium sensitized, chronic berylliosis, and PS (Liao 2021, PMID:
33602861), peripheral blood samples from sarcoidosis (Garman 2020, PMID: 33363531), and
lesional and nonlesional skin tissue from sarcoidosis (Krausgruber 2023, PMID: 36750099) for a
total of 122 patients.
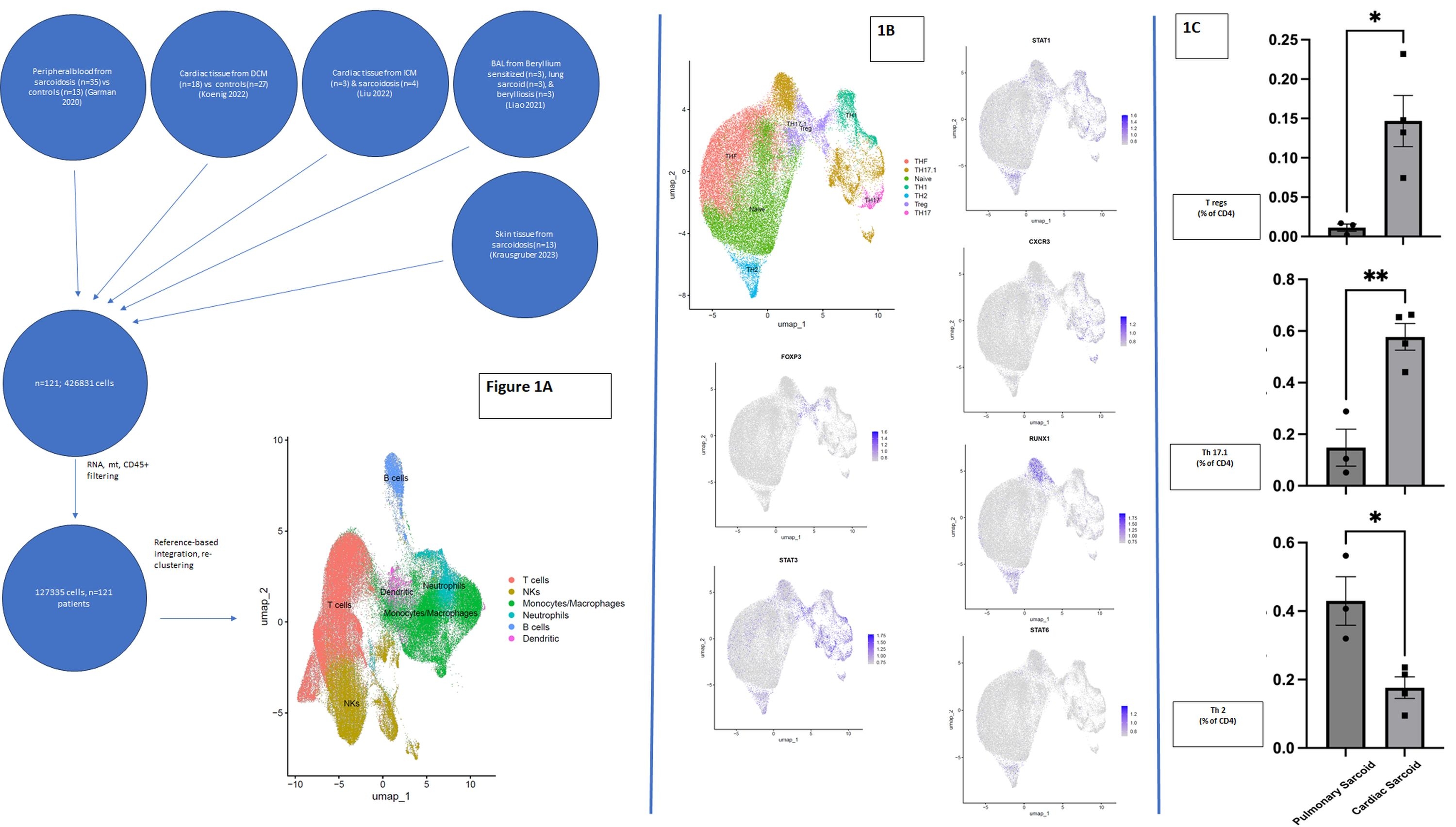
All integrative analysis was performed using the R package Seuratv5 on Stanford’s computing
cluster, Sherlock. After quality control filtering, a total of 127,335 cells were integrated using
Seurat’s reference-based integration. After labeling the UMAP with general immune cells (figure
1A), we subsetted T cells into CD4+ and CD8+ and examined the landscape of CD4+ T cells
(figure 1B).
Results
We found significantly more Tregs in CS than PS (P=0.013, figure 1C). Additionally, patients
with CS had significantly more Th17.1 cells in CS in comparison to PS (P=0.0041). However,
significantly less Th2 cells were found in CS than PS patients (P=0.0152). These differences
remained statistically significant across various analyses comparing Tregs and Th17.1 cell as a
proportion of CD4+, CD3+, and CD45+ cells.
Conclusions
In comparison to patients with PS, pathways which differentiate naive CD4+ cells into TH17.1
and Tregs are upregulated among CS patients while the TH2 differentiation pathway is
downregulated.
Our findings indicate distinct CD4+ T cell patterns among CS and PS patients. Our ongoing
work aims to further evaluate T cell proliferation and expression to elucidate mechanistic
pathways as potential drug targets for CS.
Introduction
Cardiac sarcoidosis (CS) is a life-threatening auto-immune disorder that causes the formation of
non-caseating granulomas in cardiac tissue. Th17.1 cell have been found to be upregulated in
patients with pulmonary sarcoidosis (PS) although data is lacking among CS patients.
This meta-analysis integrates all publicly available granuloma single-cell and single-nuclei RNA
sequencing data to identify patterns in immune cell activation across different granulomatous
diseases while focusing on CD4+T cell activation among CS patients.
Methods
Five studies were included, spanning cardiac tissue from healthy controls and DCM (Koenig
2022, PMID:35959412), cardiac tissue from ICM and cardiac sarcoidosis (Liu 2022,
PMID:36111531), BAL from beryllium sensitized, chronic berylliosis, and PS (Liao 2021, PMID:
33602861), peripheral blood samples from sarcoidosis (Garman 2020, PMID: 33363531), and
lesional and nonlesional skin tissue from sarcoidosis (Krausgruber 2023, PMID: 36750099) for a
total of 122 patients.
All integrative analysis was performed using the R package Seuratv5 on Stanford’s computing
cluster, Sherlock. After quality control filtering, a total of 127,335 cells were integrated using
Seurat’s reference-based integration. After labeling the UMAP with general immune cells (figure
1A), we subsetted T cells into CD4+ and CD8+ and examined the landscape of CD4+ T cells
(figure 1B).
Results
We found significantly more Tregs in CS than PS (P=0.013, figure 1C). Additionally, patients
with CS had significantly more Th17.1 cells in CS in comparison to PS (P=0.0041). However,
significantly less Th2 cells were found in CS than PS patients (P=0.0152). These differences
remained statistically significant across various analyses comparing Tregs and Th17.1 cell as a
proportion of CD4+, CD3+, and CD45+ cells.
Conclusions
In comparison to patients with PS, pathways which differentiate naive CD4+ cells into TH17.1
and Tregs are upregulated among CS patients while the TH2 differentiation pathway is
downregulated.
Our findings indicate distinct CD4+ T cell patterns among CS and PS patients. Our ongoing
work aims to further evaluate T cell proliferation and expression to elucidate mechanistic
pathways as potential drug targets for CS.
More abstracts on this topic:
Activator protein 1 (AP-1) Complex in Antigen Presenting Cells contributes to Salt-Sensitive Blood Pressure in Humans
Ahmad Taseer, Desta Selam, Kirabo Annet, Saleem Mohammad, Mutchler Ashley Pitzer, Ertuglu Lale, Albritton Claude, Haynes Alexandria, Sheng Quanhu, Khan Mohd, Demirci Mert
ApoB-100 peptide nanoparticles inhibit established atherosclerosis progression in female HLA-A*0201 transgenic miceZhou Jianchang, Zhao Xiaoning, Dimayuga Paul, Lio Nicole, Cercek Bojan, Trac Noah, Chung Eun Ji, Shah Prediman, Chyu Kuang-yuh