Final ID: Mo101
3-Mercaptopyruvate Sulfurtransferase is a Critical Regulator of Branched-Chain Amino Acid Catabolism in Cardiometabolic HFpEF
Abstract Body: Background: Heart failure with preserved ejection fraction (HFpEF) is among the leading causes of cardiovascular mortality and morbidity. Recent multi-omics data sets underscore severely impaired branched-chain amino acid (BCAA) catabolism in the failing hearts of HFpEF patients. 3-mercaptopyruvate sulfurtransferase (3-MST) is a mitochondrial hydrogen sulfide (H2S) synthase that regulates mitochondrial biogenesis, function, and substrate metabolism.
Research Questions and Goals: In the current study, we aimed to elucidate the role of 3-MST in myocardial BCAA catabolism in cardiometabolic HFpEF.
Methods: 3-MST knockout or wild-type (WT) mice were subjected to control or L-NAME and high fat diet - induced HFpEF. Echocardiographic measurements, invasive hemodynamic assessment, and exercise capacity testing were conducted to determine HFpEF severity. Metabolomic analysis was performed to study BCAA catabolism. Additionally, a H2S donor, SG1002 was administered to increase H2S bioavailability in the setting of 3-MST deficiency and HFpEF.
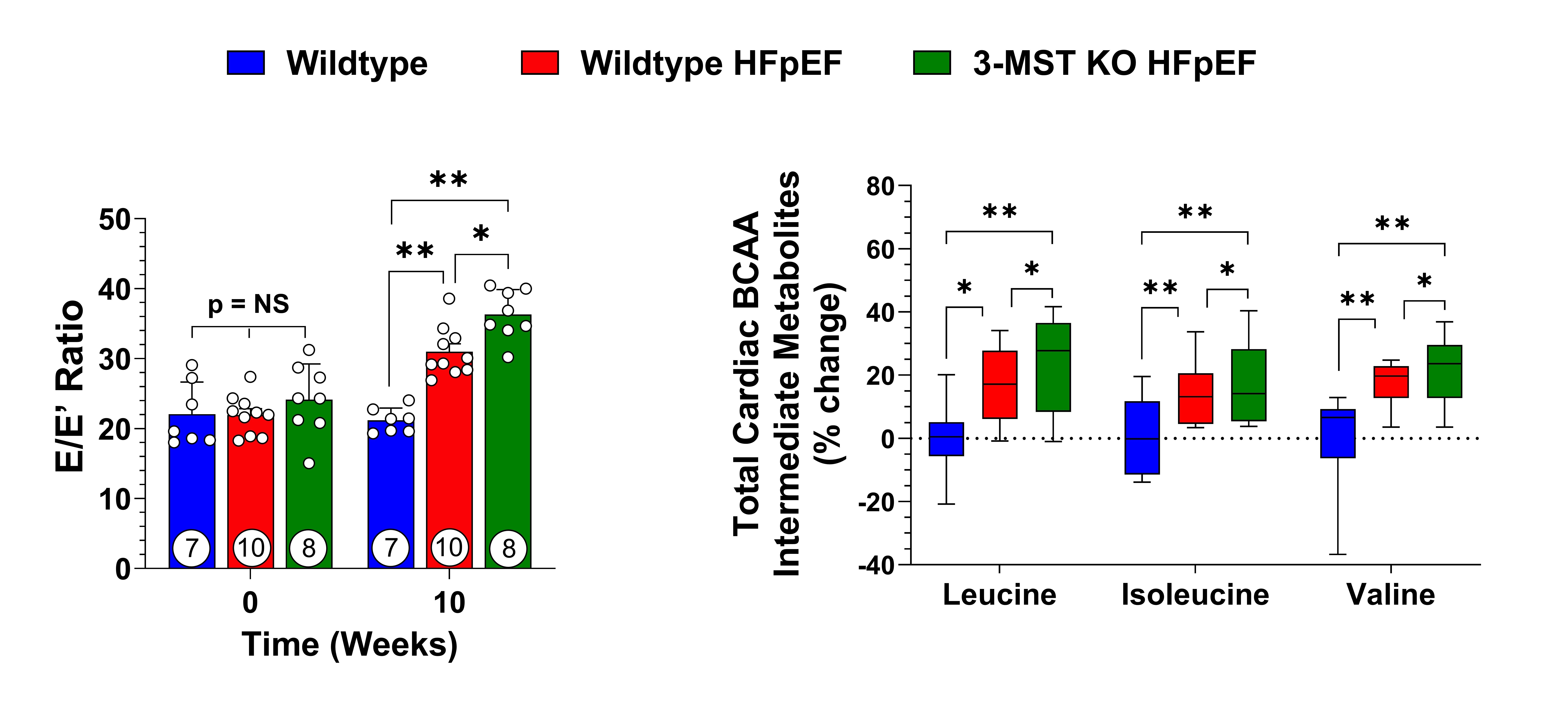
Results: We observed progressive deterioration in left ventricular (LV) diastolic function and exercise performance from mice subjected to HFpEF. Genetic ablation of 3-MST further-exacerbated HFpEF severity as evidenced by significantly increased E/e’ ratio, elevated LV end-diastolic pressure, and reduced exercise performance. Metabolomic analysis revealed pathological accumulation of intermediate metabolites of BCAA in the HFpEF hearts, suggesting a major derangement in myocardial BCAA catabolism. 3-MST KO hearts exhibited further BCAA catabolic defects as compared to those from WT. Importantly, H2S donor treatment significantly attenuated BCAA catabolic defect and alleviated HFpEF severity in both WT and 3-MST KO HFpEF mice.
Conclusion: Our results demonstrate that deficiency of 3-MST impairs myocardial BCAA catabolism, which results in exacerbated HFpEF severity in a “two-Hit” mouse model of HFpEF. These data suggest 3-MST is critically involved in physiological substrate metabolism within the mitochondria and exerts beneficial effects in HFpEF. Future experiments are underway to delineate the mechanisms by which 3-MST regulates BCAA catabolism.
Research Questions and Goals: In the current study, we aimed to elucidate the role of 3-MST in myocardial BCAA catabolism in cardiometabolic HFpEF.
Methods: 3-MST knockout or wild-type (WT) mice were subjected to control or L-NAME and high fat diet - induced HFpEF. Echocardiographic measurements, invasive hemodynamic assessment, and exercise capacity testing were conducted to determine HFpEF severity. Metabolomic analysis was performed to study BCAA catabolism. Additionally, a H2S donor, SG1002 was administered to increase H2S bioavailability in the setting of 3-MST deficiency and HFpEF.
Results: We observed progressive deterioration in left ventricular (LV) diastolic function and exercise performance from mice subjected to HFpEF. Genetic ablation of 3-MST further-exacerbated HFpEF severity as evidenced by significantly increased E/e’ ratio, elevated LV end-diastolic pressure, and reduced exercise performance. Metabolomic analysis revealed pathological accumulation of intermediate metabolites of BCAA in the HFpEF hearts, suggesting a major derangement in myocardial BCAA catabolism. 3-MST KO hearts exhibited further BCAA catabolic defects as compared to those from WT. Importantly, H2S donor treatment significantly attenuated BCAA catabolic defect and alleviated HFpEF severity in both WT and 3-MST KO HFpEF mice.
Conclusion: Our results demonstrate that deficiency of 3-MST impairs myocardial BCAA catabolism, which results in exacerbated HFpEF severity in a “two-Hit” mouse model of HFpEF. These data suggest 3-MST is critically involved in physiological substrate metabolism within the mitochondria and exerts beneficial effects in HFpEF. Future experiments are underway to delineate the mechanisms by which 3-MST regulates BCAA catabolism.
More abstracts on this topic:
A short version of HFD/L-NAME mouse model enabling time-effective proof of concept studies to evaluate drugs targeting the cardiometabolic and mild hypertension associated HFpEF phenotype.
Assaly Rana, Dubroca Caroline, Waget Aurelie, Perrier Kevin, Sulpice Thierry
A High Salt Diet Drives Kidney Microvascular Dysfunction Through a Plasma-Derived Factor that Increases Mitochondrial Reactive Oxygen SpeciesReynolds Lance, Guan Zhengrong, Pollock David, Pollock Jennifer