Final ID: We006
Metabolic Reprogramming to Increase Mitochodnrial Mass, Fusion and Energetics Represents an Important Rate Limiting Step in Direct Cardiac Reprogramming
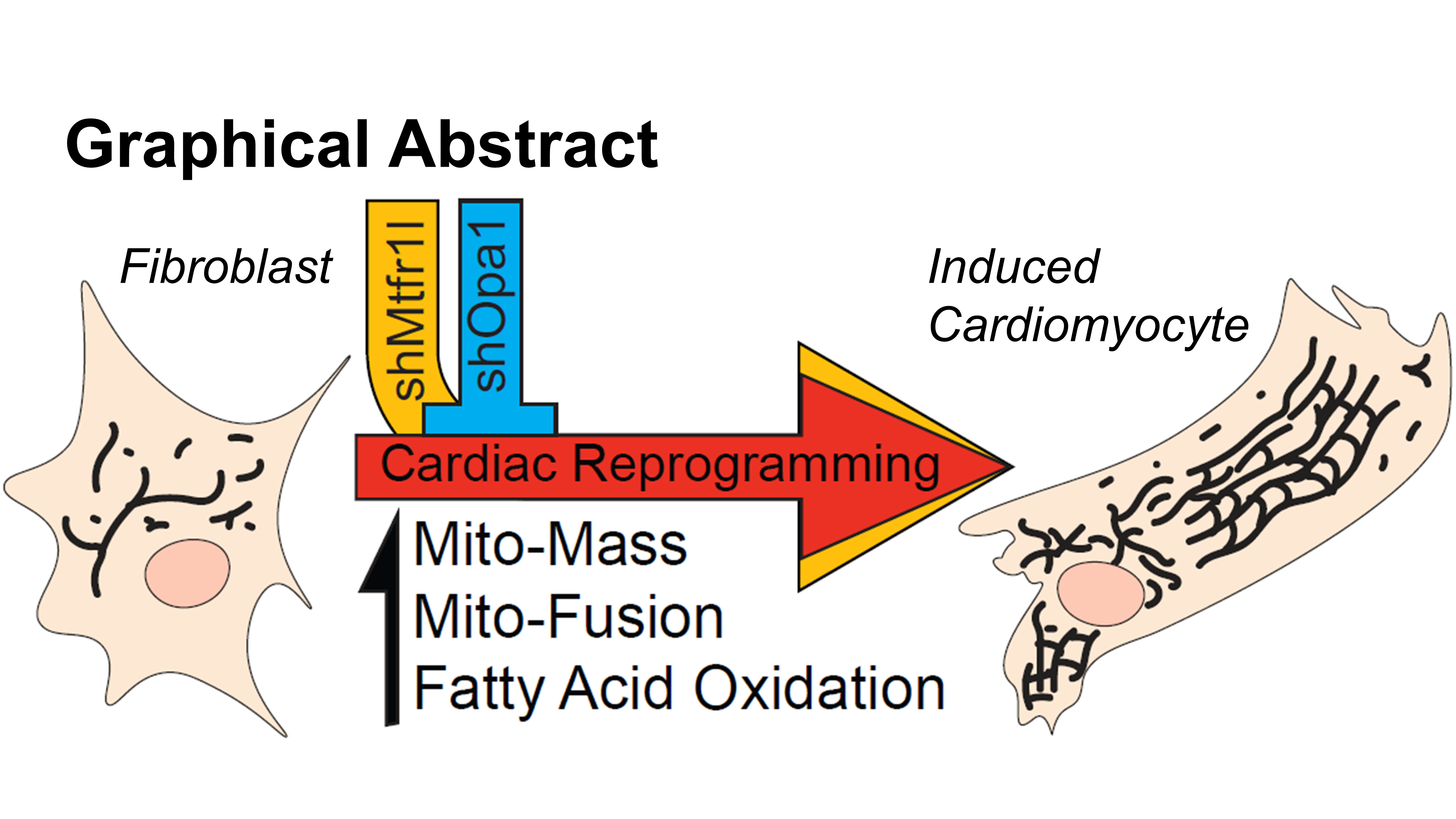
Abstract Body: Direct reprogramming of cardiac fibroblasts (CFs) into induced cardiomyocytes (iCMs) shows promise for treating myocardial infarction (MI). However, low conversion efficiency and relative immaturity of iCMs have so far precluded its clinical translation. Native CM maturation and cell cycle exit is associated with a switch from glycolytic metabolism to mitochondrial fatty acid oxidation (FAO), accompanied by a shift in the dynamic balance of mitochondrial fission (dividing) and fusion (joining) toward fusion. Because CFs lack the energy requirements of CMs, we hypothesized that transdifferentiation of CFs to iCMs requires a similar switch. Combining confocal microscopy with metabolic flux assays, we directly compared native CF and CM mitochondrial morphology and energetics. CMs showed significantly higher mitochondrial mass, fusion and energetics, all metrics relevant for increased reliance on mitochondrial FAO. Applying these techniques to study reprogramming, we found that at later reprogramming time points key metrics move significantly toward the phenotypes of native CMs. We then hypothesized that facilitating metabolic reprogramming by inhibiting fission and/or promoting fusion could improve iCM conversion. A loss of function screen identified certain fission genes, particularly the little studied mitochondrial fission regulator 1-like protein (Mtfr1l), as potent barriers to iCM conversion and maturation. In contrast, the core fusion genes were necessary for successful generation of iCMs, particularly the key fusion effector optical atrophy 1 (Opa1). Mtfr1l has been shown to promote cleavage of Opa1, and indeed concurrent knockdown of Opa1 ablated improvements to reprogramming efficiency. In conclusion, we showed both that iCMs are associated with increased mitochondrial mass, fusion and energetics approaching those of native CMs and that inducing CM-like mitochondria by knocking down Mtfr1l dramatically improved reprogramming, potentially through increased fusion activity of Opa1. By incorporating metabolic reprogramming into traditional direct cardiac reprogramming strategies, we may be able to move the field closer to clinical application to improve outcomes after MI.
More abstracts on this topic:
Adenine accumulation in Endothelial Cells Induces Cardiotoxicity in Type 2 Diabetes
Nasiripour Somayyeh, Khan Muhammad S., Tamayo Ian, Sharma Kumar, Bopassa Jean Chrisostome
Cardiac Macrophage are Key Immune Drivers of Cardiometabolic HFpEFGoel Mehak, Prabhu Sumanth, Ismahil Ameen, Sonkar Ravi, Bansal Shyam, Zhu Yujie, Liu Wei, Hamid Tariq, Rokosh Gregg, Katsnelson Michael