Final ID:
High-Dose vs. Standard-Dose Influenza Vaccine and Cardiovascular Outcomes: The FLUNITY-HD Pooled Analysis
While influenza vaccination has been shown to reduce the incidence of major adverse CV events, immune response towards standard-dose inactivated influenza vaccines (SD-IIV) is often insufficient in older adults. The high-dose inactivated influenza vaccine (HD-IIV) was specifically designed for older adults and has demonstrated superior protection against laboratory-confirmed influenza infection versus SD-IIV; however, data regarding its effectiveness among individuals with CV disease (CVD) and against CV outcomes are mainly from observational studies or specific high-risk groups.
Methods
FLUNITY-HD was a prespecified, individual-level pooled analysis of two methodologically harmonized pragmatic, individually randomized trials comparing HD-IIV vs. SD-IIV among adults aged ≥65 years (DANFLU-2, Denmark) and 65-79 years (GALFLU, Spain). Both trials used routine healthcare databases as primary data source. This prespecified analysis assessed severe CV and respiratory outcomes overall and according to history of CVD, occurring from 14 days after vaccination through May 31 the following year.
Results
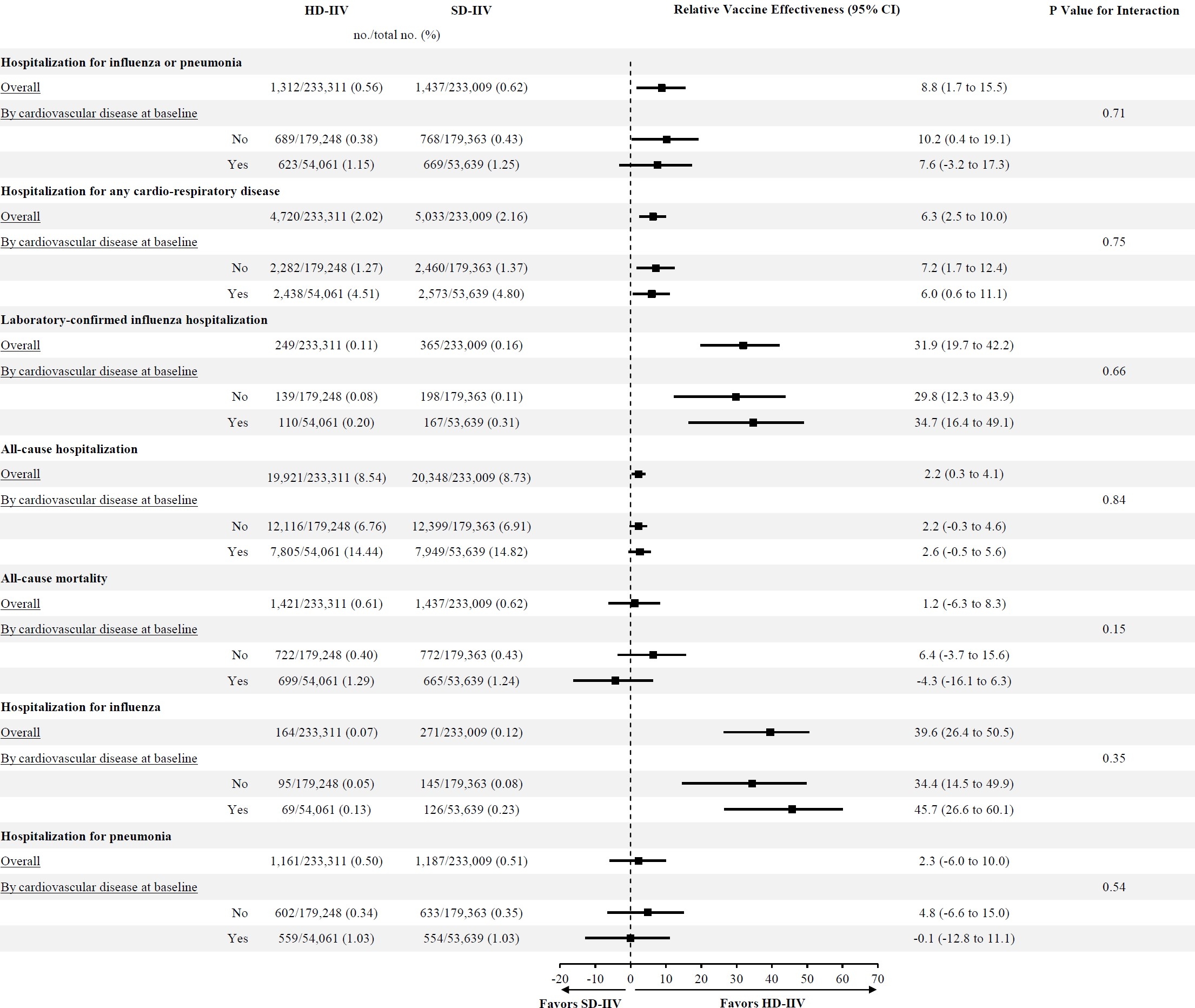
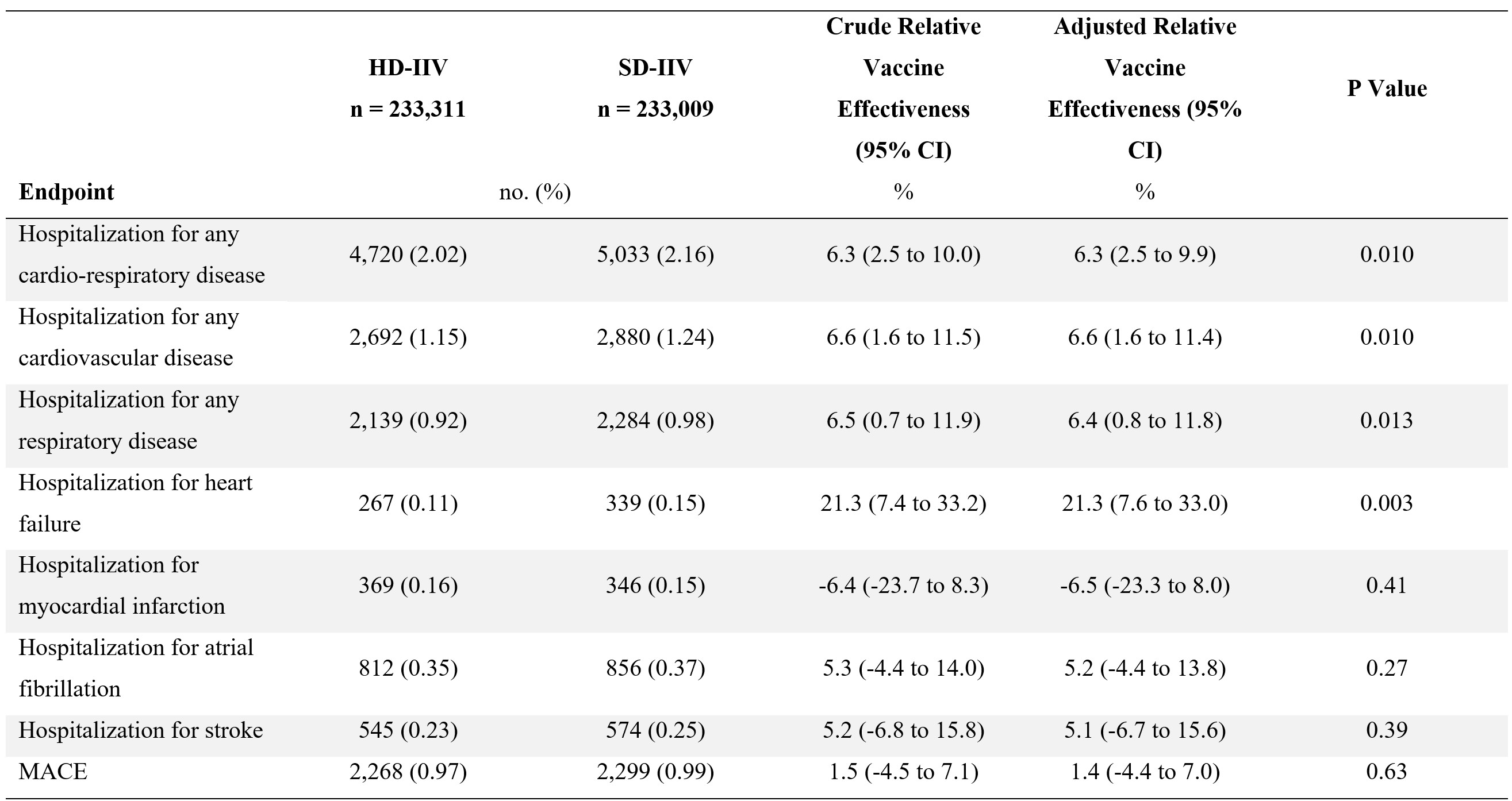
A total of 466,320 randomized participants were included in this analysis (48.0% female, mean age 73.3±5.4years), of which 107,700 (23.1%) had a history of CV. Compared with SD-IIV, HD-IIV significantly reduced the incidence of hospitalization for influenza or pneumonia, laboratory-confirmed influenza hospitalization, cardio-respiratory hospitalization, and all-cause hospitalization, with consistent effects irrespective of history of CVD (Figure 1). The incidence of hospitalization for any cardiovascular disease was lower in the HD-IIV group compared with the SD-IIV group (HD-IIV, 1.15% vs. SD-IIV, 1.24%; rVE, 6.6%; 95% CI, 1.6 to 11.4; P=0.010) (Figure 2). Participants randomized to HD-IIV also had a lower incidence of hospitalization for heart failure (HD-IIV, 0.11% vs. SD-IIV, 0.15%; rVE, 21.3%; 95% CI, 7.6 to 33.0; P=0.003).
Conclusions
In a prespecified pooled analysis of 466,320 individually randomized older adults, HD-IIV reduced the incidence of a wide range of severe CV and respiratory outcomes compared with SD-IIV, regardless of prior history of CVD. Among CV outcomes, the protective effect of HD-IIV vs. SD-IIV was particularly pronounced against hospitalizations for heart failure.
- Johansen, Niklas ( Herlev and Gentofte Hospital , Hellerup , Denmark )
- Larsen, Carsten ( Aarhus University Hospital , Aarhus , Denmark )
- Larsen, Lykke ( Odense University Hospital, Denmark , Odense C , Denmark )
- Wiese, Lothar ( Zealand University Hospital , Roskilde , Denmark )
- Dalager-pedersen, Michael ( Aalborg University Hospital , Aalborg , Denmark )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Janstrup, Kira Hyldekær ( Herlev and Gentofte Hospital , Hellerup , Denmark )
- Duran Parrondo, Carmen ( Xunta de Galicia , Santiago de Compostela , Spain )
- Pineiro-sotelo, Marta ( Xunta de Galicia , Santiago de Compostela , Spain )
- Cribeiro-gonzalez, Martin ( Xunta de Galicia , Santiago de Compostela , Spain )
- Conde-pajaro, Monica ( Xunta de Galicia , Santiago de Compostela , Spain )
- Modin, Daniel ( Herlev and Gentofte Hospital , Hellerup , Denmark )
- Miras-carballal, Susana ( Xunta de Galicia , Santiago de Compostela , Spain )
- Gonzalez Perez, Juan-manuel ( Xunta de Galicia , Santiago de Compostela , Spain )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Sivapalan, Pradeesh ( Herlev and Gentofte Hospital , Hellerup , Denmark )
- Martel, Cyril ( Statens Serum Institut , Copenhagen , Denmark )
- Jensen, Jens-ulrik Stæhr ( Herlev and Gentofte Hospital , Hellerup , Denmark )
- Martinon-torres, Federico ( Hospital Clinico Universitario de Santiago de Compostela , Santiago de Compostela , Spain )
- Biering-srensen, Tor ( Herlev and Gentofte Hospital , Hellerup , Denmark )
- Pardo-seco, Jacobo ( Instituto de Investigación Sanitaria de Santiago de Compostela , Santiago de Compostela , Galicia , Spain )
- Rodriguez-tenreiro, Carmen ( Instituto de Investigación Sanitaria de Santiago de Compostela , Santiago de Compostela , Galicia , Spain )
- Loiacono, Matthew ( Sanofi Vaccines , Morristown , New Jersey , United States )
- Harris, Rebecca ( Sanofi , Lyon , France )
- Dufournet, Marine ( Sanofi , Lyon , France )
- Van Aalst, Robertus ( Sanofi Vaccines , Morristown , New Jersey , United States )
- Chit, Ayman ( Sanofi Vaccines , Morristown , New Jersey , United States )
Meeting Info:
Session Info:
More abstracts on this topic:
Matshela Mamotabo
A New Biomarker of Aging Derived From Electrocardiogram Improves Risk Prediction of Incident Myocardial Infarction and Stroke.Wilsgaard Tom, Rosamond Wayne, Schirmer Henrik, Lindekleiv Haakon, Attia Zachi, Lopez-jimenez Francisco, Leon David, Iakunchykova Olena
More abstracts from these authors:
Modin Daniel, Janstrup Kira Hyldekær, Larsen Carsten, Larsen Lykke, Wiese Lothar, Dalager-pedersen Michael, Kober Lars, Solomon Scott, Sivapalan Pradeesh, Jensen Jens-ulrik Stæhr, Martel Cyril, Johansen Niklas, Krause Tyra Grove, Biering-srensen Tor, Angrist Joshua D., Yeh Robert, Wadhera Rishi, Vaduganathan Muthiah, Bhatt Ankeet, Chatur Safia, Claggett Brian
Effectiveness of High-Dose vs. Standard-Dose Inactivated Influenza Vaccine in Individuals with and without Atherosclerotic Cardiovascular Disease: A Pooled FLUNITY-HD Trial AnalysisPareek Manan, Chit Ayman, Larsen Carsten, Larsen Lykke, Wiese Lothar, Dalager-pedersen Michael, Claggett Brian, Janstrup Kira Hyldekær, Duran Parrondo Carmen, Pineiro-sotelo Marta, Cribeiro-gonzalez Martin, Johansen Niklas, Conde-pajaro Monica, Miras-carballal Susana, Gonzalez Perez Juan-manuel, Solomon Scott, Sivapalan Pradeesh, Martel Cyril, Jensen Jens-ulrik Stæhr, Martinon-torres Federico, Biering-srensen Tor, Modin Daniel, Pardo-seco Jacobo, Rodriguez-tenreiro Carmen, Loiacono Matthew, Harris Rebecca, Dufournet Marine, Van Aalst Robertus