Final ID: LBP2
Targeting Neuroinflammation After Cardiac Arrest Using Anti-Inflammatory Lipid Nanoparticles
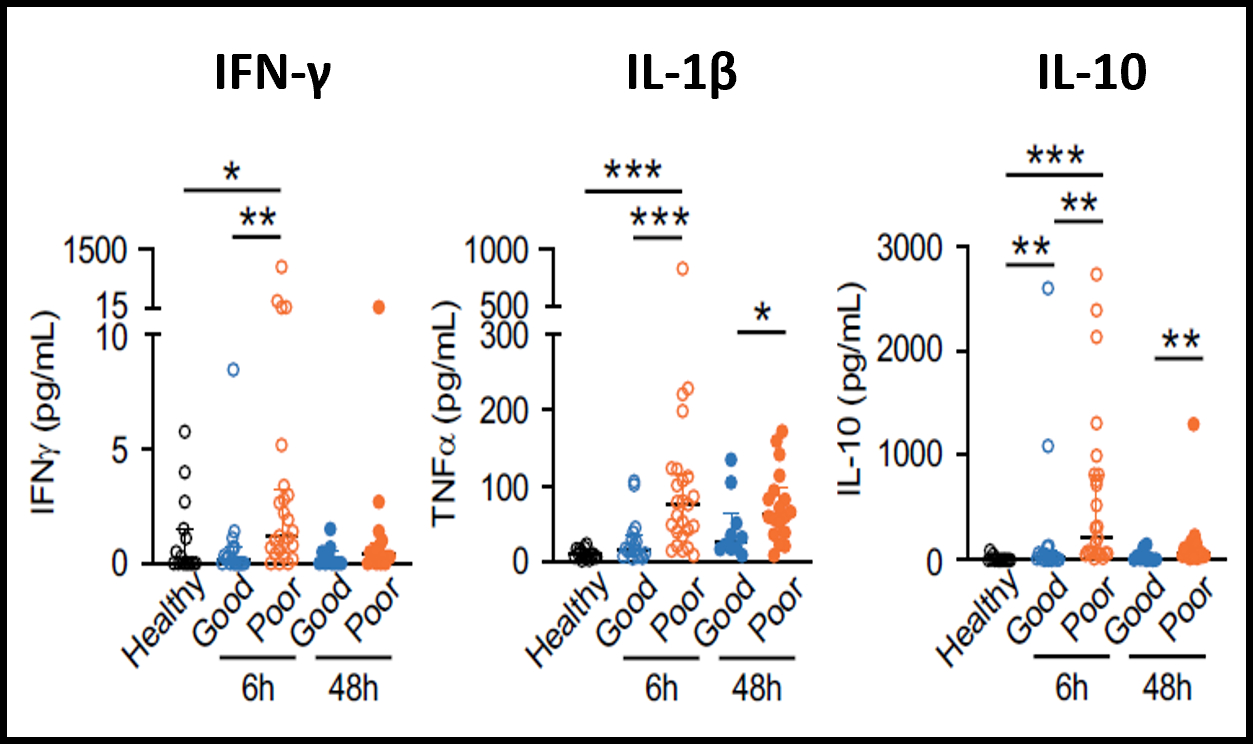
Abstract Body (Do not enter title and authors here): Background: Out-of-hospital cardiac arrest (OHCA) leads to ischemia-reperfusion injury (IRI), systemic and neuroinflammation, neuronal damage, and mortality. In our previous study, patients with poor neurological outcomes at 30 days post-OHCA showed elevated levels of IL-10 and proinflammatory cytokines such as TNF-α and IFN-γ, suggesting that endogenous IL-10 is a compensatory but inadequate response to systemic inflammation (Fig. 1). Our objective is to develop a cell-based therapeutic strategy that enhances endogenous anti-inflammatory responses. We hypothesized that lipid nanoparticles (LNP) delivering IL-10 mRNA to monocytes would drive anti-inflammatory programs in the brain and reduce neurological injury after cardiac arrest.
Methods: Mice were subjected to 10 minutes of CA followed by resuscitation. IL-10 mRNA (0.7 mg/kg) was encapsulated in monocyte-targeted LNPs and administered intravenously (i.v.) just after return of spontaneous circulation (ROSC). Neurological scores were assessed before euthanasia. Blood and tissues (brain, liver, spleen) were collected 24 hours post-CA for cytokine analysis and flow cytometric assessment.
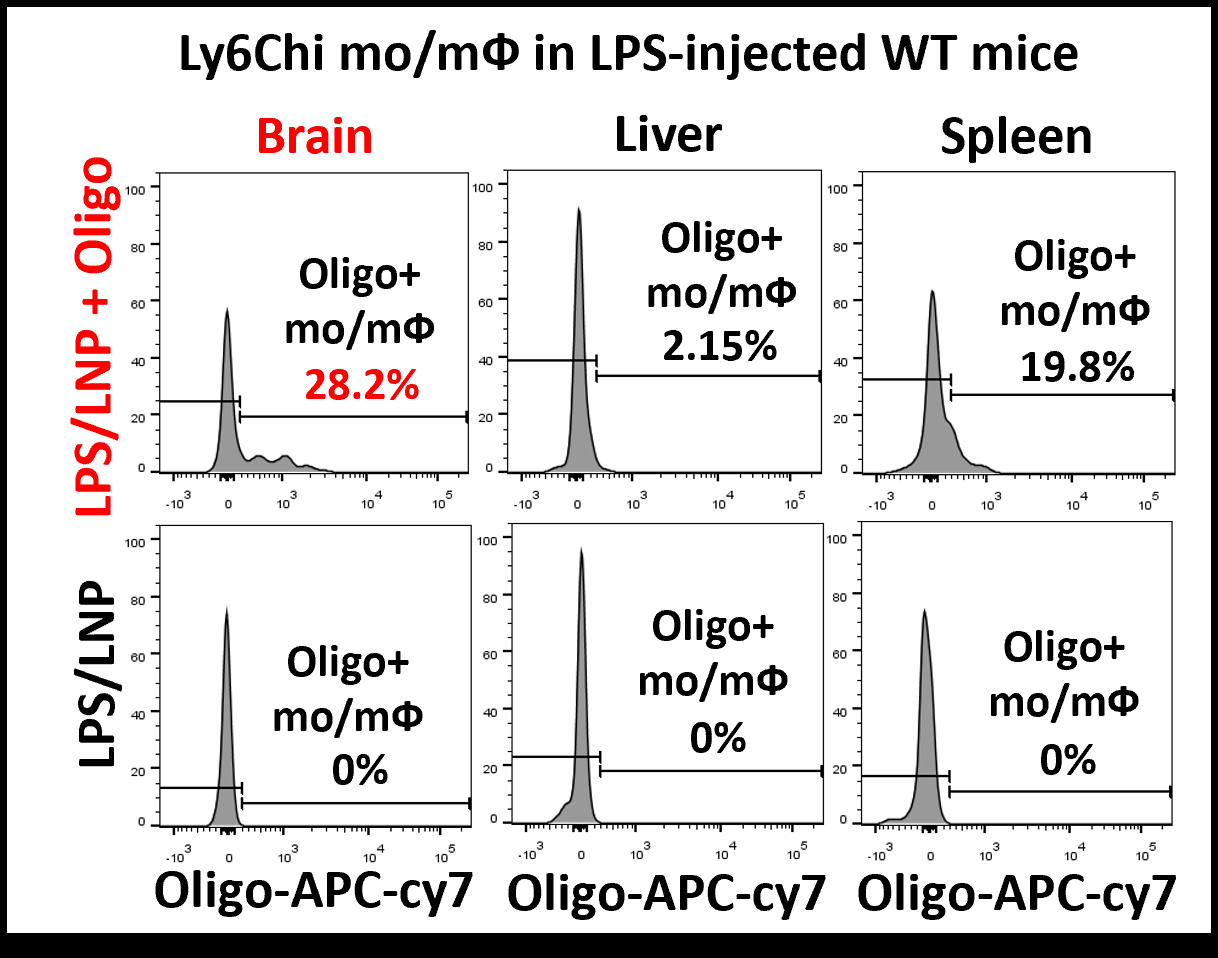
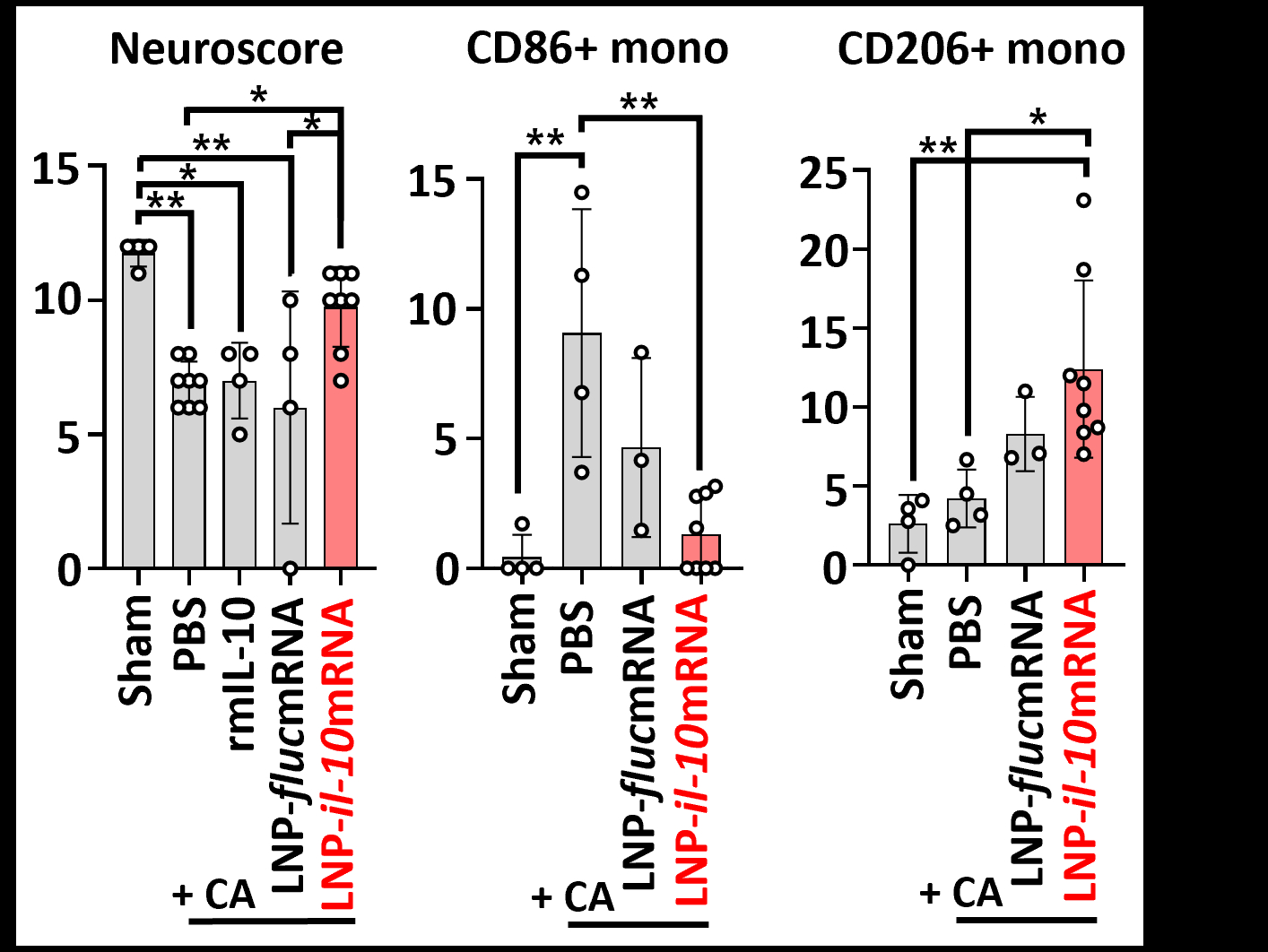
Results: During the development of monocyte-targeted LNP, biodistribution analysis showed increased uptake of LNP-encapsulated reporter oligonucleotides by monocytes that migrated to the brain in a murine model of endotoxemia. In the murine model of CA, treatment with LNP-il10mRNA i.v. significantly improved post-CA neurological scores compared to PBS, LNP enclosing control mRNA, or recombinant IL-10 (20 ug) i.v. 24h post-CA, LNP-il0mRNA significantly reduced the relative abundance of inflammatory Ly6c(hi)CD86+ monocytes in the brain (PBS treatment 9.1%; LNP-il10mRNA 1.3%) and increased the abundance of anti-inflammatory Ly6c(low)CD206+ monocytes (PBS treatment 4.2%; LNP-il10mRNA 12.4%) (Fig. 3).
Conclusion: Monocyte-targeted delivery of IL-10 mRNA via LNPs effectively enhanced anti-inflammatory signaling and reprogramed immune cell phenotypes after murine CA. This approach improved neurological outcomes and overcame limitations of systemic recombinant IL-10 therapy by enabling cell-specific and brain-directed immunomodulation. These results support the therapeutic potential of LNP-based mRNA delivery for “cell-based therapy” to mitigate neuroinflammation and improve recovery after CA.
Conflicts of Interest: None
Keywords: lipid nanoparticle, il10mRNA, monocyte modulation, post-cardiac arrest-derived brain injury (PCABI)
Methods: Mice were subjected to 10 minutes of CA followed by resuscitation. IL-10 mRNA (0.7 mg/kg) was encapsulated in monocyte-targeted LNPs and administered intravenously (i.v.) just after return of spontaneous circulation (ROSC). Neurological scores were assessed before euthanasia. Blood and tissues (brain, liver, spleen) were collected 24 hours post-CA for cytokine analysis and flow cytometric assessment.
Results: During the development of monocyte-targeted LNP, biodistribution analysis showed increased uptake of LNP-encapsulated reporter oligonucleotides by monocytes that migrated to the brain in a murine model of endotoxemia. In the murine model of CA, treatment with LNP-il10mRNA i.v. significantly improved post-CA neurological scores compared to PBS, LNP enclosing control mRNA, or recombinant IL-10 (20 ug) i.v. 24h post-CA, LNP-il0mRNA significantly reduced the relative abundance of inflammatory Ly6c(hi)CD86+ monocytes in the brain (PBS treatment 9.1%; LNP-il10mRNA 1.3%) and increased the abundance of anti-inflammatory Ly6c(low)CD206+ monocytes (PBS treatment 4.2%; LNP-il10mRNA 12.4%) (Fig. 3).
Conclusion: Monocyte-targeted delivery of IL-10 mRNA via LNPs effectively enhanced anti-inflammatory signaling and reprogramed immune cell phenotypes after murine CA. This approach improved neurological outcomes and overcame limitations of systemic recombinant IL-10 therapy by enabling cell-specific and brain-directed immunomodulation. These results support the therapeutic potential of LNP-based mRNA delivery for “cell-based therapy” to mitigate neuroinflammation and improve recovery after CA.
Conflicts of Interest: None
Keywords: lipid nanoparticle, il10mRNA, monocyte modulation, post-cardiac arrest-derived brain injury (PCABI)
More abstracts on this topic:
Altered inflammatory state and mitochondrial function identified by transcriptomics in paediatric congenital heart patients prior to surgical repair
Bartoli-leonard Francesca, Harris Amy, Caputo Massimo
BXOS110 for Acute Ischemic Stroke Treatment (BEST): A Multicenter, Double-Blind, Randomized, Placebo-Controlled, Phase 2 TrialDing Yarong, Li Shuya, Wang Zhe, Li Zixiao, Meng Xia, Zhao Xingquan, Wang Yongjun, Li Zixiao