Final ID:
Efficacy and safety of flecainide compared to amiodarone for atrial fibrillation cardioversion in patients with Coronary Artery Disease and preserved ejection fraction. FLECA-ED trial
Abstract Body (Do not enter title and authors here): Hypothesis and Purpose: The FLECA-ED trial (NCT05549752) is the first randomized clinical study evaluating whether intravenous flecainide is safe and more effective than amiodarone for pharmacological cardioversion of atrial fibrillation (AF) in the emergency department (ED) setting in patients with coronary artery disease (CAD), preserved left ventricular ejection fraction and absence of residual ischemia.
Study Design and Methods: This multicenter, open-label, randomized controlled trial enrolled adult patients presenting to the ED with symptomatic paroxysmal AF. Participants were randomized 1:1 to receive intravenous flecainide or amiodarone (Tsioufis P et al, J Clin Med 2023;12(12):3961).
Population Studied: Eligible patients had documented CAD and AF duration <24 hours (or <7 days if anticoagulated with NOACs for >30 days). Patients with anginal symptoms, acute coronary syndrome or equivocal ischemia were excluded.
Interventions: After informed consent, patients were monitored with a 24-hour Holter device. Flecainide was administered at 2.0 mg/kg (maximum 150 mg), while amiodarone was given at 5.0–7.0 mg/kg over 1 hour, followed by a continuous infusion of 50 mg/h (maximum 1000 mg over 24 hours). Patients achieving sinus rhythm (SR) within 6 hours were discharged. Holter data were analyzed for complex ventricular arrhythmias and bradycardic events.
Power Calculations: The trial was powered (85% power; α = 0.05) to detect a difference in cardioversion rates, assuming 65% efficacy for flecainide and 23% for amiodarone. A total of 60 patients in the interim analysis was required.
Primary Endpoints: Restoration of SR at 2 and 6 hours; proarrhytmic events.
Secondary Endpoints: Time to SR within 24 h; bradycardic events.
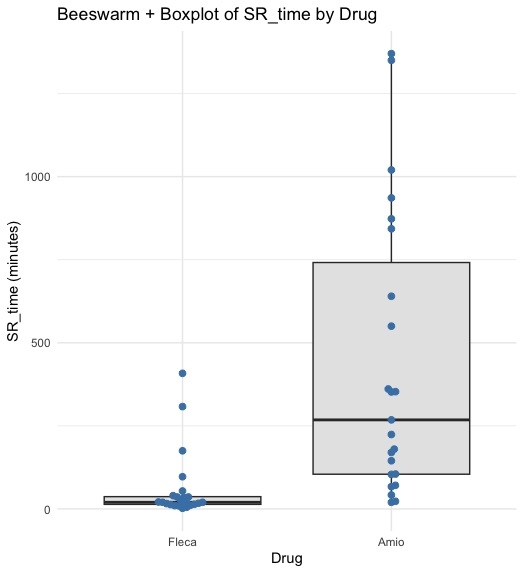
Outcomes: At 2 hours, cardioversion was achieved in 21 patients (72.4%) in the flecainide group vs. 8 (22.2%) in the amiodarone group (p < 0.001). At 6 hours, SR was restored in 23 patients (79.3%) on flecainide compared to 15 (41.6%) on amiodarone (p = 0.05). Flecainide resulted in a significantly shorter time to conversion (see Figure). No complex ventricular tachyarrhythmias were observed in either group.
Conclusions: In this interim analysis of the FLECA-ED trial, flecainide was as safe and more superior to amiodarone for pharmacological cardioversion in ED patients with paroxysmal AF and stable CAD, challenging traditional contraindications and supporting the reevaluation of flecainide use in this population. Full results will be presented
Study Design and Methods: This multicenter, open-label, randomized controlled trial enrolled adult patients presenting to the ED with symptomatic paroxysmal AF. Participants were randomized 1:1 to receive intravenous flecainide or amiodarone (Tsioufis P et al, J Clin Med 2023;12(12):3961).
Population Studied: Eligible patients had documented CAD and AF duration <24 hours (or <7 days if anticoagulated with NOACs for >30 days). Patients with anginal symptoms, acute coronary syndrome or equivocal ischemia were excluded.
Interventions: After informed consent, patients were monitored with a 24-hour Holter device. Flecainide was administered at 2.0 mg/kg (maximum 150 mg), while amiodarone was given at 5.0–7.0 mg/kg over 1 hour, followed by a continuous infusion of 50 mg/h (maximum 1000 mg over 24 hours). Patients achieving sinus rhythm (SR) within 6 hours were discharged. Holter data were analyzed for complex ventricular arrhythmias and bradycardic events.
Power Calculations: The trial was powered (85% power; α = 0.05) to detect a difference in cardioversion rates, assuming 65% efficacy for flecainide and 23% for amiodarone. A total of 60 patients in the interim analysis was required.
Primary Endpoints: Restoration of SR at 2 and 6 hours; proarrhytmic events.
Secondary Endpoints: Time to SR within 24 h; bradycardic events.
Outcomes: At 2 hours, cardioversion was achieved in 21 patients (72.4%) in the flecainide group vs. 8 (22.2%) in the amiodarone group (p < 0.001). At 6 hours, SR was restored in 23 patients (79.3%) on flecainide compared to 15 (41.6%) on amiodarone (p = 0.05). Flecainide resulted in a significantly shorter time to conversion (see Figure). No complex ventricular tachyarrhythmias were observed in either group.
Conclusions: In this interim analysis of the FLECA-ED trial, flecainide was as safe and more superior to amiodarone for pharmacological cardioversion in ED patients with paroxysmal AF and stable CAD, challenging traditional contraindications and supporting the reevaluation of flecainide use in this population. Full results will be presented
More abstracts on this topic:
A novel risk score predicts the prevalence of left atrial low-voltage areas and rhythm outcome in patients undergoing long-standing persistent atrial fibrillation ablation
Ooka Hirotaka, Nakao Sho, Kusuda Masaya, Ariyasu Wataru, Kudo Satoshi, Fujii Subaru, Mano Toshiaki, Matsuda Yasuhiro, Masuda Masaharu, Okamoto Shin, Ishihara Takayuki, Nanto Kiyonori, Tsujimura Takuya, Hata Yosuke, Uematsu Hiroyuki
A novel reproducible low-cost model of acute myocardial infarction in swineLi Yichen, Zheng Zilong, Tang Weijie, Chen Wangping, Yang Jinfu, Fan Chengming