Final ID: LBP24
A Highly Selective and Orally Available HDAC6 Inhibitor, EKZ-102, Ameliorates Cardiac Dysfunction and Exercise Intolerance in Cardiometabolic HFpEF
Abstract Body (Do not enter title and authors here): Background: HFpEF is a complex and prevalent condition with few effective treatments. HDAC6 regulates several biological processes, including autophagy, mitochondrial function and inflammation, through deacetylation of non-histone proteins, but its impact and therapeutic potential in HFpEF have not been thoroughly evaluated. We investigated the efficacy of a highly potent and selective small molecule HDAC6 inhibitor, EKZ-102, in a "two-hit" HFpEF mouse model. EKZ-102 possesses several advantageous characteristics including a next-generation oxadiazole Zn2+-binding domain, low nanomolar activity, remarkable selectivity for HDAC6 over all other HDAC paralogs (>1,000-fold), favorable bioavailability with the potential for once daily oral dosing, and CNS penetrance. Furthermore, EKZ-102 exhibits a clean in vitro safety profile and no observed adverse effects levels (NOAELs) have been identified in rodent and non-rodent species in 28-day GLP toxicology studies, poising EKZ-102 for clinical assessment.
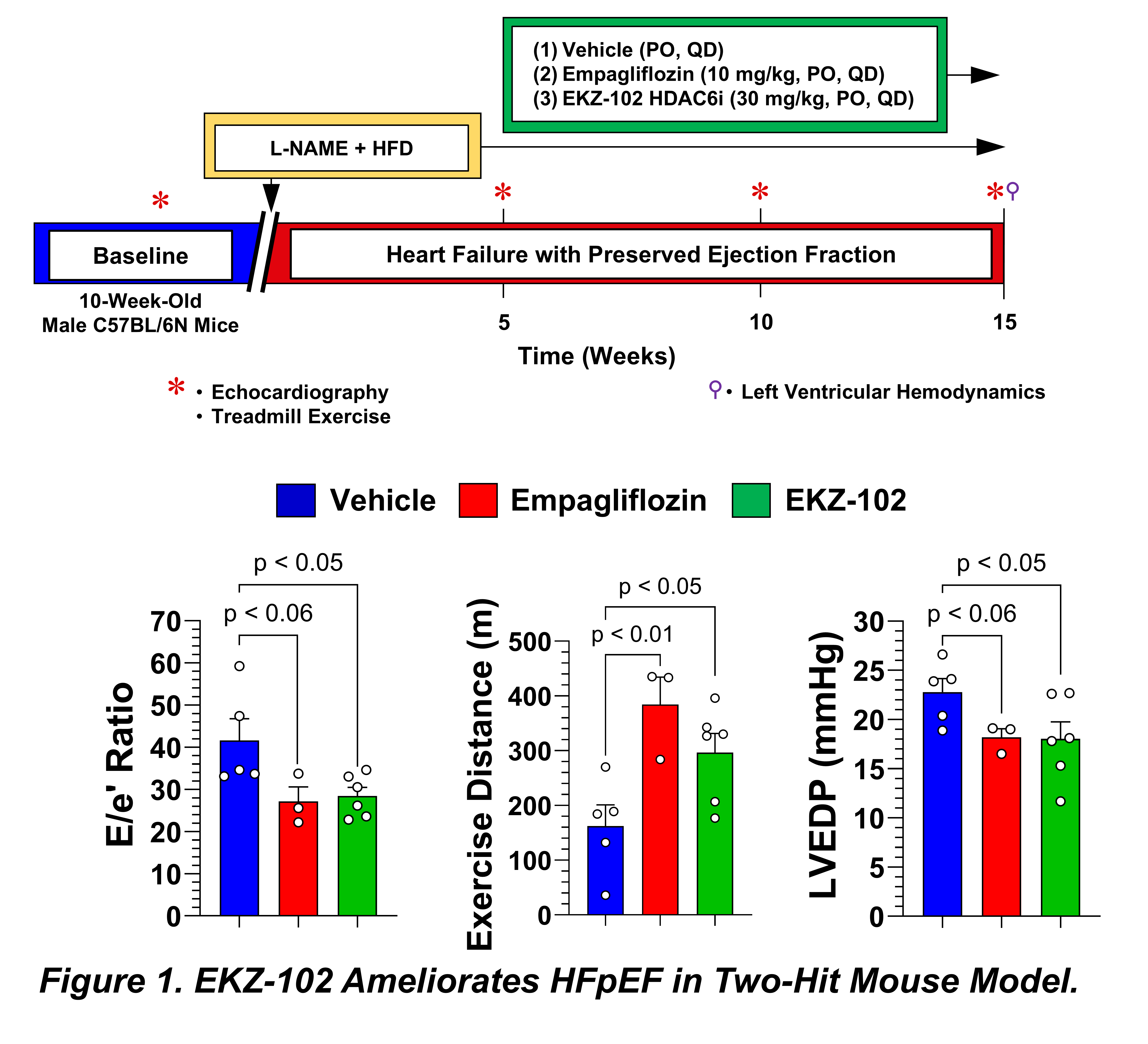
Methods: HFpEF was induced in 10-week-old male C57BL/6N mice using HFD combined with the NOS inhibitor L-NAME (1.5 g/kg). After 5 weeks, mice were randomized to receive daily oral gavage of either vehicle (1% CMC), empagliflozin (10 mg/kg), or EKZ-102 (30 mg/kg) for 10 weeks. We assessed the effects of treatment on body weight, echocardiographic indices of cardiac function, treadmill exercise capacity, and invasive systemic and LV pressures.
Results: Echocardiography confirmed preserved LVEF across all groups. Following 10 weeks of treatment, HDAC6 inhibition significantly reduced the E/e' ratio compared to HFpEF controls, with effects comparable to the clinically approved SGLT2 inhibitor treatment, empagliflozin. Vehicle-treated mice exhibited deteriorated treadmill exercise running distance and work; notably, HDAC6 inhibition with EKZ-102 significantly improved both of these clinically relevant markers, mirroring the improvements seen with empagliflozin. While no treatment affected systemic blood pressure, EKZ-102 treatment significantly reduced LVEDP compared to vehicle controls, a benefit not statistically significant with empagliflozin.
Conclusion: Our findings demonstrate that pharmacological inhibition of HDAC6 with EKZ-102 improves key pathological and functional deficits in a well-established mouse model of HFpEF. These results highlight HDAC6 as a promising therapeutic target for HFpEF and warrant further investigation into EKZ-102's clinical potential.
Methods: HFpEF was induced in 10-week-old male C57BL/6N mice using HFD combined with the NOS inhibitor L-NAME (1.5 g/kg). After 5 weeks, mice were randomized to receive daily oral gavage of either vehicle (1% CMC), empagliflozin (10 mg/kg), or EKZ-102 (30 mg/kg) for 10 weeks. We assessed the effects of treatment on body weight, echocardiographic indices of cardiac function, treadmill exercise capacity, and invasive systemic and LV pressures.
Results: Echocardiography confirmed preserved LVEF across all groups. Following 10 weeks of treatment, HDAC6 inhibition significantly reduced the E/e' ratio compared to HFpEF controls, with effects comparable to the clinically approved SGLT2 inhibitor treatment, empagliflozin. Vehicle-treated mice exhibited deteriorated treadmill exercise running distance and work; notably, HDAC6 inhibition with EKZ-102 significantly improved both of these clinically relevant markers, mirroring the improvements seen with empagliflozin. While no treatment affected systemic blood pressure, EKZ-102 treatment significantly reduced LVEDP compared to vehicle controls, a benefit not statistically significant with empagliflozin.
Conclusion: Our findings demonstrate that pharmacological inhibition of HDAC6 with EKZ-102 improves key pathological and functional deficits in a well-established mouse model of HFpEF. These results highlight HDAC6 as a promising therapeutic target for HFpEF and warrant further investigation into EKZ-102's clinical potential.
More abstracts on this topic:
A RETRO-ENANTIOMER OF ANGIOTENSIN-(1-9) PREVENTS THE DEVELOPMENT OF HEART FAILURE WITH PRESERVED EJECTION FRACTION.
Ocaranza Maria Paz, Jimenez Veronica, Yanez Osvaldo, Jalil Jorge, Venegas Camilo, Candia Camila, Hermoso Marcela, Gabrielli Luigi, Morales Javier, Oyarzun Felipe, Torres Cristian, Lillo Pablo
Aberrant Trans- and De- Nitrosylation Underpins Nitrosative Stress in Cardiometabolic HFpEFLi Zhen, Borch Jensen Martin, Vondriska Thomas, Lefer David, Gehred Natalie, Gromova Tatiana, Lapenna Kyle, Sharp Thomas, Chen Jingshu, Shambhu Smitha, Yu Xiaoman, Goodchild Traci