Final ID:
Two Mechanistically Distinct Factor XI Antibodies, REGN9933 and REGN7508, for the Prevention of Venous Thromboembolism After Knee Arthroplasty: the ROXI-VTE-I and ROXI-VTE-II trials
Methods: ROXI-VTE-I (NCT05618808) and ROXI-VTE-II (NCT06454630) are randomized, open-label phase 2 studies conducted in patients undergoing knee arthroplasty. In ROXI-VTE-I, 373 patients were randomized to receive 300-mg REGN9933 (single IV dose), 40-mg enoxaparin (SC once daily), or 2.5-mg apixaban (orally twice daily). In ROXI-VTE-II, 179 patients were randomized to receive 250-mg REGN7508 (single IV dose) or 40-mg enoxaparin (SC once daily). All treatments started 12–24 hours post-surgery. The prespecified primary endpoint was objectively confirmed VTE. REGN9933/REGN7508 was considered superior to enoxaparin if the posterior probability (pp) of log odds ratio (OR) was >95%. Cross-study pooled analyses (prespecified for REGN7508; post-hoc for REGN9933) employed an 8.6% noninferiority margin. The principal safety outcome was bleeding.
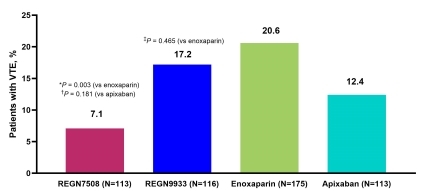
Results: In ROXI-VTE-I, the VTE rates were 17.2% (20/116), 22.2% (26/117), and 12.4% (14/113) with REGN9933, enoxaparin, and apixaban, respectively. For REGN9933 vs enoxaparin, the mean adjusted OR (90% credible interval) was 0.78 (0.47–1.32); the pp was 79%. In ROXI-VTE-II, the VTE rates were 7.1% (8/113) and 17.2% (10/58) with REGN7508 and enoxaparin, respectively. For REGN7508 vs combined enoxaparin arms, the mean adjusted OR (90% credible interval) was 0.37 (0.20–0.68); the pp was >99%. The cross-study pooled analyses (Figure 1) showed that the VTE risk difference (95% CI) for REGN7508 vs enoxaparin was −13.6% (−21.1% to −6.0%; P=0.003), REGN7508 vs apixaban was −5.3% (−13.2% to 2.4%; P=0.181), and REGN9933 vs enoxaparin was −3.5% (−12.7% to 5.7%; P=0.465). No major or clinically relevant nonmajor bleeds occurred.
Conclusions: In the prespecified analysis, REGN7508 was superior to enoxaparin for VTE prevention. The cross-study analyses showed REGN7508 was noninferior to apixaban and REGN9933 was noninferior to enoxaparin.
- Weitz, Jeffrey ( MCMASTER UNIVERSITY , Hamilton , Ontario , Canada )
- Olenchock, Benjamin ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Gutstein, David ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Segers, Annelise ( Itreas , Amsterdam , Netherlands )
- Roberts, Robin ( MCMASTER UNIVERSITY , Hamilton , Ontario , Canada )
- Bonaca, Marc ( CPC Clinical Research , Aurora , Colorado , United States )
- Raskob, Gary ( UNIV OF OKLAHOMA , Oklahoma City , Oklahoma , United States )
- Kithcart, Aaron ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- O'brien, Meagan ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Levy, Oren ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Marin, Ethan ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Onisko, Malgorzata ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Mohammadi, Kusha ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Li, Dateng ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
- Meagher, Karoline ( Regeneron Pharmaceuticals, Inc. , Tarrytown , New York , United States )
Meeting Info:
Session Info:
Arteries and Veins in Trouble: VTE and PAD
Saturday, 11/08/2025 , 09:45AM - 11:00AM
Featured Science
More abstracts on this topic:
Al Said Samer, Goodman Shaun, Joung Boyoung, Kiss Robert, Spinar Jindrich, Wojakowski Wojciech, Weitz Jeffrey, Bloomfield Dan, Sabatine Marc, Ruff Christian, Patel Siddharth, Giugliano Robert, Morrow David, Goodrich Erica, Murphy Sabina, Hug Bruce, Parker Sanobar, Chen Shih-ann
A Novel Anti-Thrombotic and Anti-AF Drug without an Adverse Bleeding ProblemRicchiuti Nikola, Niebrzydowski Maxwell, Richardson Abigail, Yan Jiajie, Ai Xun
More abstracts from these authors:
Young Bryan, Hirshberg Boaz, George Richard, Olenchock Benjamin, Devalaraja-narashimha Kishor, Morton Lori, Janssens Stefan, Redaelli Giulia, Mei Jingning, Kithcart Aaron, Herman Gary
Safety and Tolerability of REGN5381, a Monoclonal Antibody Agonist of NPR1 in Patients With Heart Failure With Reduced Ejection FractionYoung Bryan, Herman Gary, Olenchock Benjamin, George Richard, Hirshberg Boaz, Devalaraja-narashimha Kishor, Morton Lori, Janssens Stefan, Redaelli Giulia, Mei Jingning, Kithcart Aaron