Final ID:
Safety and Efficacy of Induced Pluripotent Stem Cell-derived Engineered Human Myocardium as Biological Ventricular Assist Tissue in Terminal Heart Failure - BioVAT-HF-DZHK20 – Phase II Interim Data Report
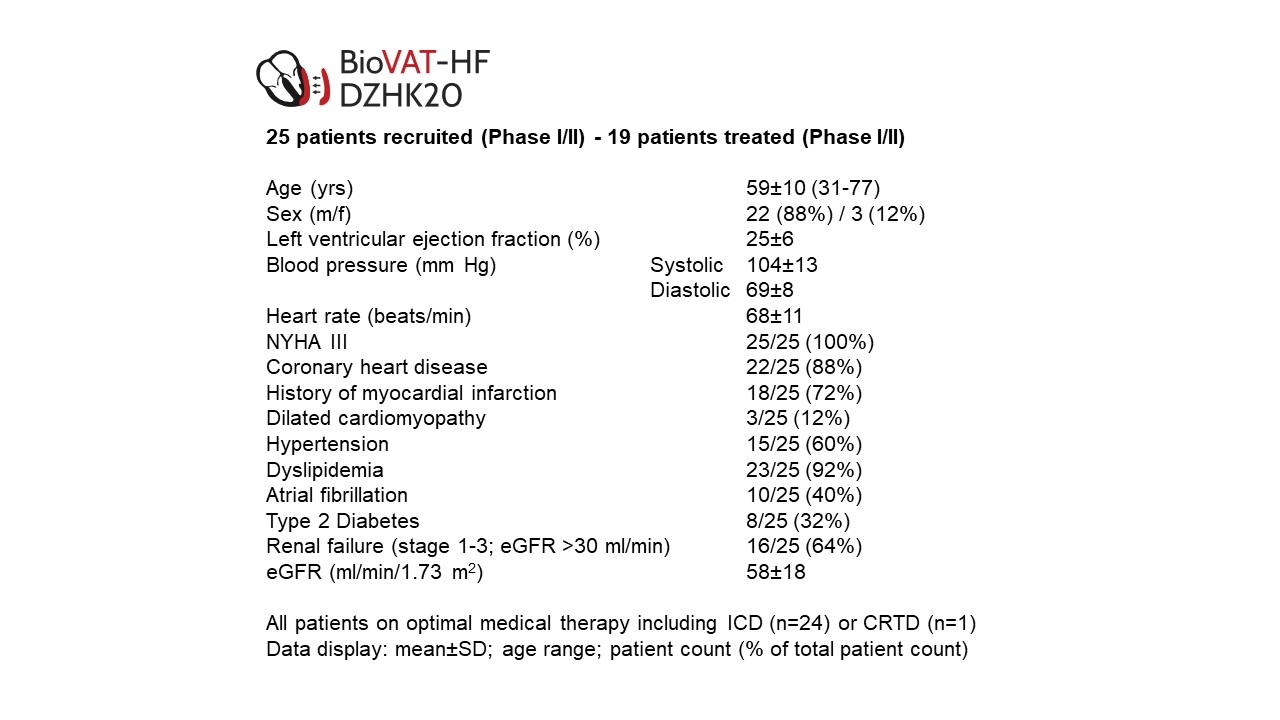
Data presented as mean±SD, count, and percent of the respective patient cohort
Statistical testing of pre-post comparison by paired, two-tailed Student’s t-test
- Ensminger, Stephan ( University Heart Center Luebeck , Luebeck , Germany )
- Fujita, Buntaro ( University Heart Center Luebeck , Luebeck , Germany )
- Gerecke, Birgit ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Hasenfuss, Gerd ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Hellenkamp, Kristian ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Herrmann-lingen, Christoph ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Jebran, Ahmad-fawad ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Jurczyk, Dominik ( University Heart Center Luebeck , Luebeck , Germany )
- Kowallick, Johannes ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Legler, Tobias ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Lotz, Joachim ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Kutschka, Ingo ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Placzek, Marius ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Puehler, Thomas ( University Heart Center Luebeck , Luebeck , Germany )
- Riggert, Joachim ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Sadlonova, Monika ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Tiburcy, Malte ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Zimmermann, Wolfram ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Paitazoglou, Christina ( University Heart Center Luebeck , Luebeck , Germany )
- Seidler, Tim ( Kerckhoff-Klinik , Bad Nauheim , Germany )
- Brandenburg, Soeren ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Anker, Stefan ( Charite , Berlin , Germany )
- Bremmer, Felix ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
- Eitel, Ingo ( University Heart Center Luebeck , Luebeck , Germany )
- Friede, Tim ( UNIVERSITY MED CTR GOETTINGEN , Goettingen , Germany )
Meeting Info:
Session Info:
Biological and Pragmatic Interventions in Heart Failure: From Present to Future
Sunday, 11/09/2025 , 08:00AM - 09:15AM
Featured Science
More abstracts on this topic:
Cao Ning, Qiu Hui, Li Hongwei
Association Between the Natriuretic Peptides-Cyclic Guanosine Monophosphate Cascade and Left Ventricular Reverse Remodeling After Acute Anterior Myocardial InfarctionArai Marina, Sawada Kenichiro, Matama Hideo, Fujino Masashi, Yoneda Shuichi, Takagi Kensuke, Takahama Hiroyuki, Otsuka Fumiyuki, Kataoka Yu, Nishimura Kunihiro, Noguchi Teruo, Asaumi Yasuhide, Minamino Naoto, Yasuda Satoshi, Honda Satoshi, Ogata Soshiro, Kiyoshige Eri, Miura Hiroyuki, Nakao Kazuhiro, Murai Kota, Iwai Takamasa
More abstracts from these authors:
Dittrich Gesine, Xu Xingbo, Riester Daniel, Meyer Tim, Tiburcy Malte, Fischer Andre, Zimmermann Wolfram
Regenerating the Human HeartZimmermann Wolfram