Final ID:
Effect of Sotatercept on Mortality and Major Morbidity Outcomes in Patients with Pulmonary Arterial Hypertension: Pooled Analysis of the PULSAR, STELLAR, and ZENITH Trials
Research Questions/Hypothesis: Analyze mortality and major morbidity outcomes during the complete DBPC periods of 3 sotatercept studies in PAH.
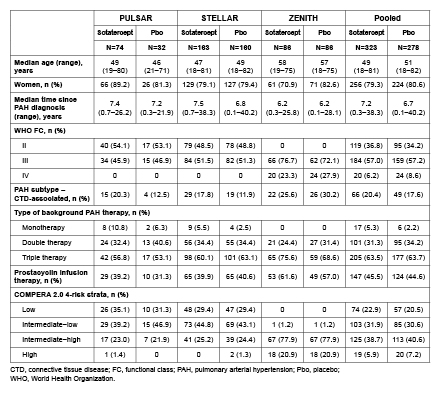
Methods: Data were pooled from pts with PAH receiving background therapy and randomized to sotatercept or pbo in PULSAR (WHO FC II/III; Phase 2; NCT03496207), STELLAR (WHO FC II/III; Phase 3; NCT04576988), and ZENITH (WHO FC III/IV; Phase 3; NCT04896008). This post-hoc analysis comprised (A) time to first morbidity event (lung transplantation, PAH worsening-related hospitalization [≥24 h]), or all-cause death, (B) transplant-free survival (TFS), and (C) overall survival (OS). Hazard ratios (HRs) and 95% confidence intervals (CIs) were generated by Cox proportional hazards models, stratified by study, and P-values by stratified log-rank tests.
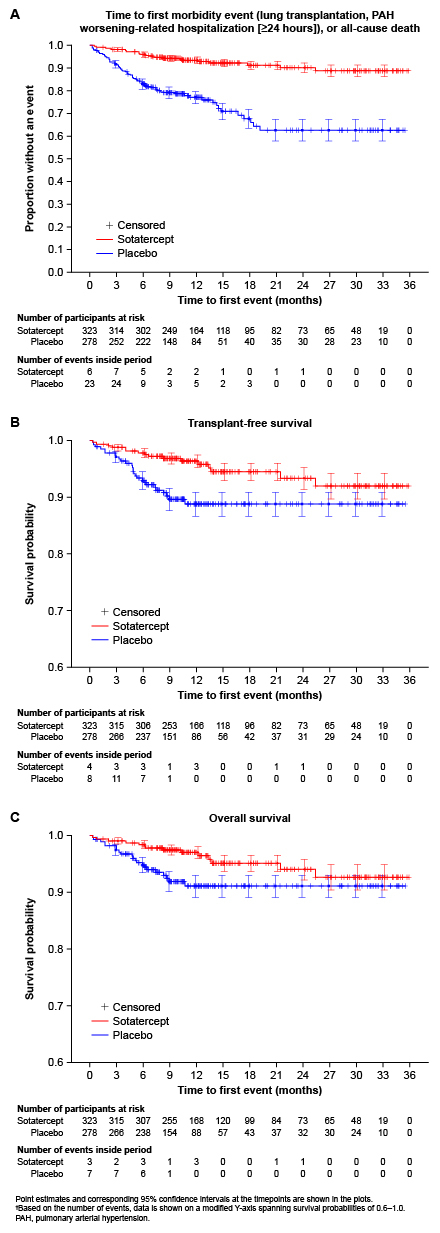
Results: The analysis included 323 pts treated with sotatercept and 278 with pbo. Baseline characteristics were generally comparable between arms, including most having had long-standing PAH (Table 1). Compared with pbo, sotatercept significantly reduced the risk of a first morbidity–mortality event (HR 0.25, 95% CI: 0.16–0.39, P<0.0001; a total of 25 pts had ≥1 event with sotatercept vs 69 pts with pbo), and improved TFS (HR 0.44, 95% CI: 0.24–0.83, P=0.0047; 16 vs 27 pts with ≥1 event) and OS (HR 0.49, 95% CI: 0.25–0.98, P=0.0192; 14 vs 21 pts with ≥1 event) (Fig. 1). By 3–6 months, all 3 Kaplan–Meier curves demonstrated clear separation between treatment arms (Fig. 1).
Conclusions: Sotatercept significantly improved a composite endpoint of mortality and major morbidity outcomes, TFS, and OS, in a pooled analysis of 3 DBPC studies in pts with PAH spanning a broad range of WHO FC, background therapies, and COMPERA risk strata.
- Mclaughlin, Vallerie ( University of Michigan Medical School , Ann Arbor , Michigan , United States )
- Miller, Barry ( Merck & Co., Inc. , Rahway , New Jersey , United States )
- Shi, Yaru ( Merck & Co., Inc. , Rahway , New Jersey , United States )
- Lin, Jianxin ( Merck & Co., Inc. , Rahway , New Jersey , United States )
- Loureiro, Maria José ( Merck & Co., Inc. , Rahway , New Jersey , United States )
- Patel, Mahesh ( Merck & Co., Inc. , Rahway , New Jersey , United States )
- Cornell, Alexandra G. ( Merck & Co., Inc. , Rahway , New Jersey , United States )
- Humbert, Marc ( University Paris Saclay , Le Kremlin Bicetre , France )
- Badesch, David ( University of Colorado , Aurora , Colorado , United States )
- Ghofrani, Ardeschir ( University Hospital Giessen , Giessen , Germany )
- Gibbs, Simon ( Imperial College London , London , United Kingdom )
- Gomberg-maitland, Mardi ( George Washington University School of Medicine and Health Sciences , Washington , District of Columbia , United States )
- Hoeper, Marius ( Hannover Medical School , Hannover , Germany )
- Preston, Ioana ( Lahey Hospital and Medical Center , Burlington , Massachusetts , United States )
- Souza, Rogerio ( University of Sao Paulo , Sao Paulo , Brazil )
- Waxman, Aaron ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
Meeting Info:
Session Info:
Arteries and Veins in Trouble: VTE and PAD
Saturday, 11/08/2025 , 09:45AM - 11:00AM
Featured Science
More abstracts on this topic:
Dubrock Hilary, Wieczorek Mikolaj, Hackett Sarah, Alger Heather, Carlson Katherine, Klugherz Paul, Carter Rickey, Wagner Tyler, Johnson Patrick, Frantz Robert, Strom Jordan, Waks Jonathan, Agarwal Richa, Hemnes Anna, Steinberg Benjamin, Pandey Ambarish
A Body Shape Index at Age 25-64 Predicts Mortality and CHD HospitalizationShafran Itamar, Krakauer Nir, Krakauer Jesse, Cohen Gali, Gerber Yariv
More abstracts from these authors:
Horn Evelyn, Weatherald Jason, Hoeper Marius
Opening the Medicine Cabinet: Current Therapeutic Strategies for PAHGomberg-maitland Mardi, Airhart Sophia, Chin Kelly