Final ID:
Improving Detection of Transthyretin Cardiac Amyloidosis with AI: A Single-Arm Multicenter Trial
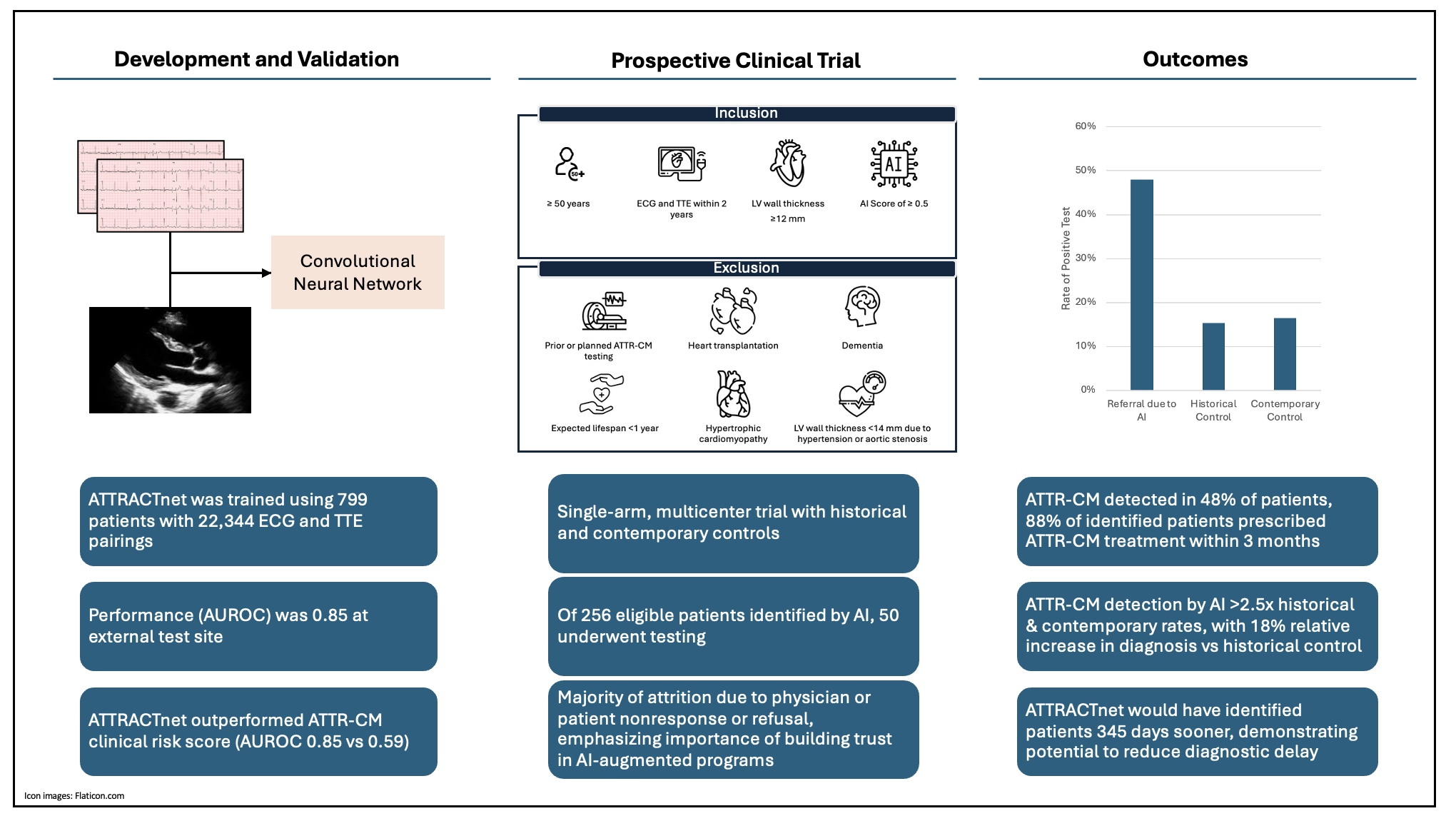
Study Design and Methods: We developed a machine learning model, ATTRACTnet, using electrocardiogram (ECG) waveforms and transthoracic echocardiogram (TTE) measurements, and conducted a multicenter, single-arm, open-label trial to evaluate its real-world performance (NCT06469372). The trial spanned eight hospitals in New York City, where eligible patients were identified by the model, notified, and offered diagnostic testing and follow-up care.
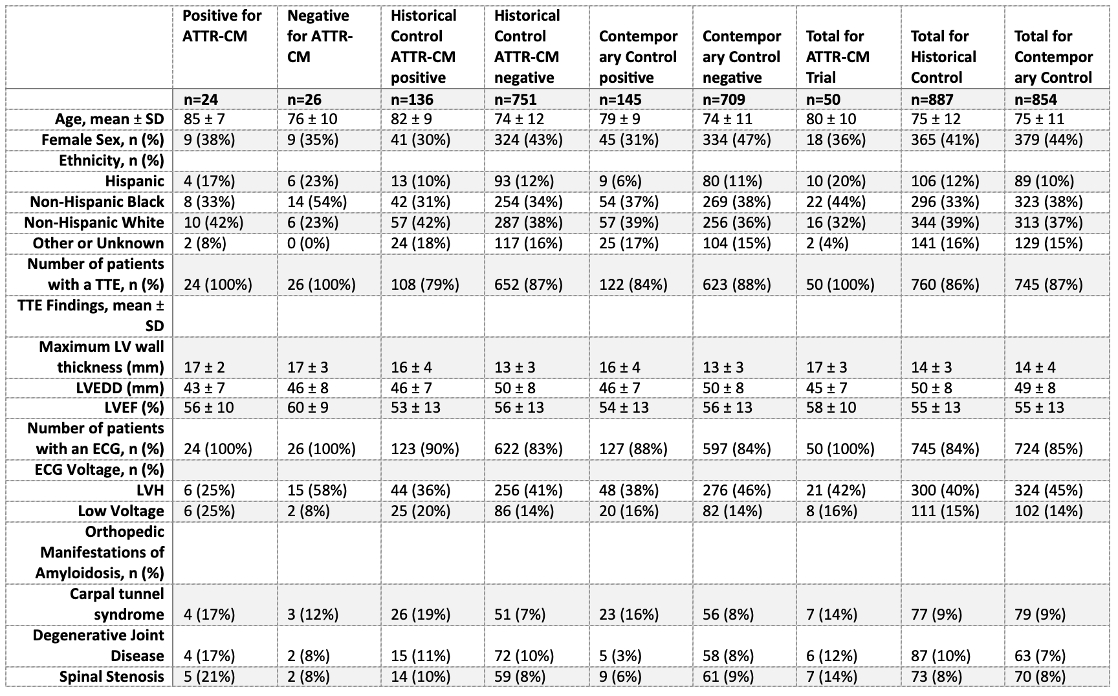
Sample Size: 799 patients contributed 22,344 ECG-TTE pairs to model development. The prospective trial enrolled 256 eligible patients, of whom 50 underwent cardiac amyloidosis testing.
Population Studied: Patients ≥ 50 years old with an ECG and TTE within 2 years with left ventricular (LV) wall thickness ≥ 12 mm who had an AI model score of ≥0.5 were eligible for inclusion in the trial. Exclusion criteria included prior ATTR-CM testing, heart transplantation, dementia, expected lifespan <1 year, hypertrophic cardiomyopathy, or LV wall thickness <14 mm explained by uncontrolled hypertension or moderate/severe aortic stenosis.
Intervention(s): Clinicians of flagged patients were contacted via EHR messaging. Upon approval, patients were offered cardiac amyloidosis scintigraphy and monoclonal protein testing at Columbia or local sites per clinician discretion.
Power Calculations: This feasibility trial used prespecified interim analyses after 25 and 50 patients.
Primary End Points: Proportion of tested patients diagnosed with ATTR-CM vs. historical and contemporary controls.
Outcome(s)[Statistical Plan or Main Results]: ATTRACTnet outperformed a validated risk score (AUROC 0.85 vs. 0.59). Of 50 tested, 24 (48%) were diagnosed with ATTR-CM, exceeding historical (15.3%) and contemporary (17.0%) rates (p<0.001), and 88% of diagnosed patients began therapy within 3 months. The model flagged patients a median of 345 days before enrollment. The trial resulted in an 18% relative increase in system-wide diagnoses compared to the prior year.
- Jain, Sneha ( Stanford University , Redwood City , California , United States )
- Brown, Kathleen ( Columbia University , New York , New York , United States )
- Ramlall, Vijendra ( Columbia University , New York , New York , United States )
- Nicholas, Nicholas ( Columbia University , New York , New York , United States )
- Elhadad, Noemie ( Columbia University , New York , New York , United States )
- Rodriguez, Fatima ( STANFORD UNIVERSITY , Palo Alto , California , United States )
- Witteles, Ronald ( STANFORD UNIVERSITY SCHOOL OF MED , Stanford , California , United States )
- Goyal, Parag ( Weill Cornell Medicine , New York , New York , United States )
- Homma, Shunichi ( Columbia University Medical Center , New York , New York , United States )
- Einstein, Andrew ( Columbia University , New York , New York , United States )
- Maurer, Mathew ( Columbia University , New York , New York , United States )
- Sun, Tony ( Columbia University , New York , New York , United States )
- Elias, Pierre ( COLUMBIA UNIVERSITY MEDICAL CENTER , New York , New York , United States )
- Poterucha, Timothy ( Mayo Clinic , Rochester , Minnesota , United States )
- Pierson, Emma ( Univeristy of California , Berkley , California , United States )
- Roedan Oliver, Francisco ( Columbia University , New York , New York , United States )
- Castillo, Michelle ( Columbia University , New York , New York , United States )
- Wan, Ningxin ( Weill Cornell Medicine , New York , New York , United States )
- Alishetti, Shudhanshu ( Weill Cornell Medicine , New York , New York , United States )
- Hartman, Heidi ( Columbia University , New York , New York , United States )
- Finer, Joshua ( NewYork-Presbyterian Hospital , Monsey , New York , United States )
Meeting Info:
Session Info:
Rewriting the Code for Cardiac Amyloid: Novel Identification, Treatment, and Cure
Monday, 11/10/2025 , 01:30PM - 02:45PM
Featured Science
More abstracts on this topic:
Stern Lily, Fine Nowell, Maurer Mathew, Grogan Martha, Ambardekar Amrut, Grodin Justin, Soman Prem, Garcia-pavia Pablo, Chen Chris, Siddhanti Suresh, Tamby Jean-francois, Fox Jonathan
β1-adrenergic autoantibodies (β1-AA) augment macropinocytosis in CD4+ T cells, leading to the expansion of CD4+CD28− T cell subsets in heart failure.Sun Fei, Yao Junyan, Li Bingjie, Zhang Suli, Liu Huirong
More abstracts from these authors:
Finer Joshua, Poterucha Timothy, Haggerty Chris, Jing Linyuan, Jain Sneha, Einstein Andrew, Maurer Matthew, Raghunath Sushravya, Rocha Daniel, Elias Pierre
AL Cardiac Amyloidosis: A High Risk, High Reward Condition with Contemporary TreatmentMaurer Mathew, Witteles Ronald