Final ID: 4377089
Clonal Hematopoiesis Associated with Trp53 and Dnmt3a Mutations Promotes Tissue Repair in Acute Cardiovascular Diseases
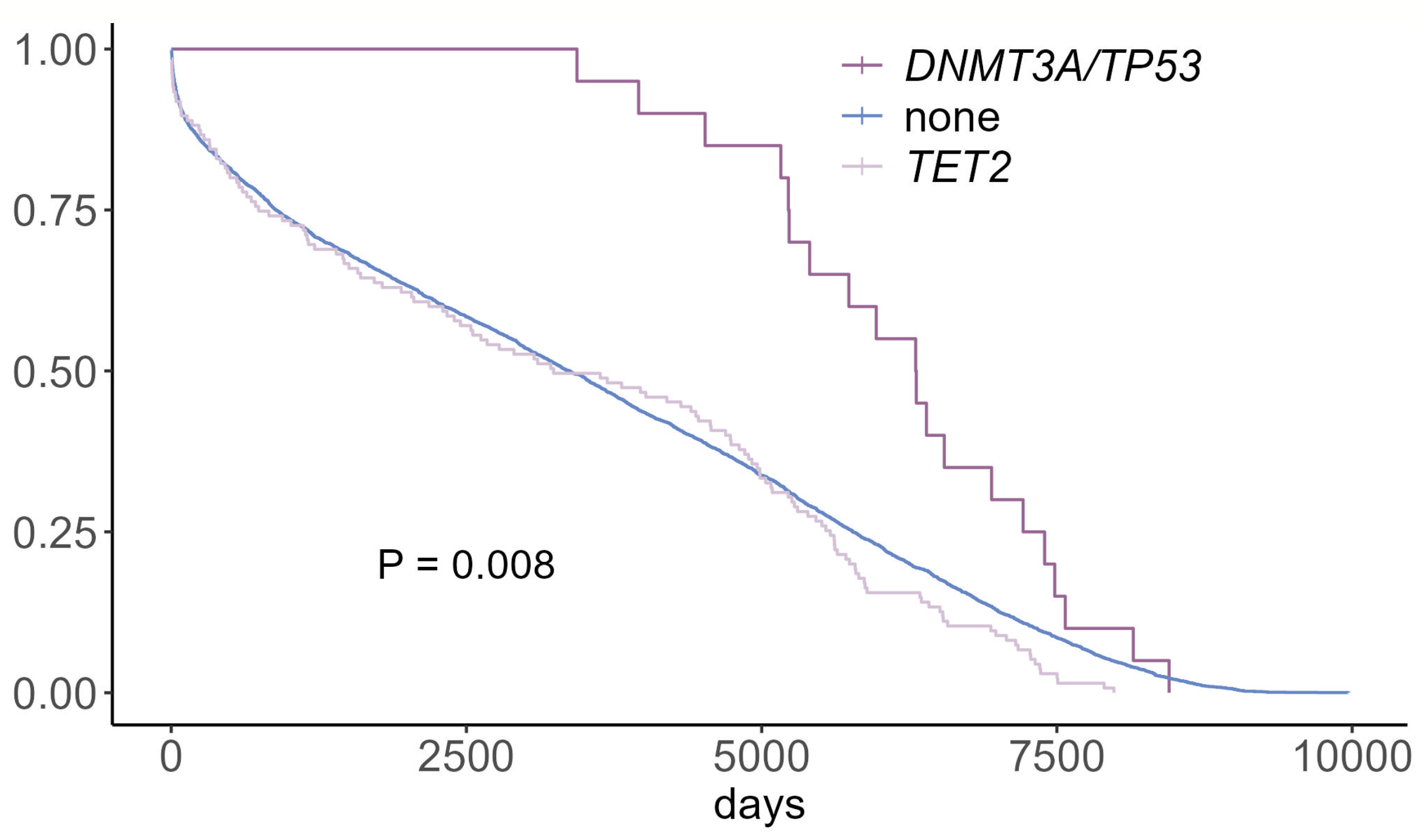
Abstract Body (Do not enter title and authors here): Introduction: Clonal hematopoiesis (CH)—the expansion of blood cell clones carrying somatic mutations (e.g., DNMT3A, TP53)—is associated with increased cardiovascular disease (CVD) risk and adverse outcomes in chronic CVD. However, its role in acute CVD remains unclear. In our analysis, patients with DNMT3A- or TP53-driven CH who experienced acute CVD exhibited reduced rehospitalization rates and improved survival, suggesting a potential pro-reparative effect.
Methods: We analyzed whole-exome sequencing data from the UK Biobank to identify CH-associated mutations in patients with acute CVD and correlated mutation status with clinical outcomes. To model CH in vivo, we transplanted ~10% bone marrow cells harboring Dnmt3a or Trp53 mutations into wild-type mice. Acute CVD models—including myocardial infarction (MI, via left anterior descending artery ligation), hind limb ischemia (HLI, via femoral artery ligation), and cardiotoxin (CTX)-induced muscle injury—were evaluated for functional and histological repair using echocardiography, histology, and fibrosis quantification. Mechanistic studies included single-cell RNA sequencing and epigenetic profiling to uncover pathways contributing to enhanced tissue regeneration.
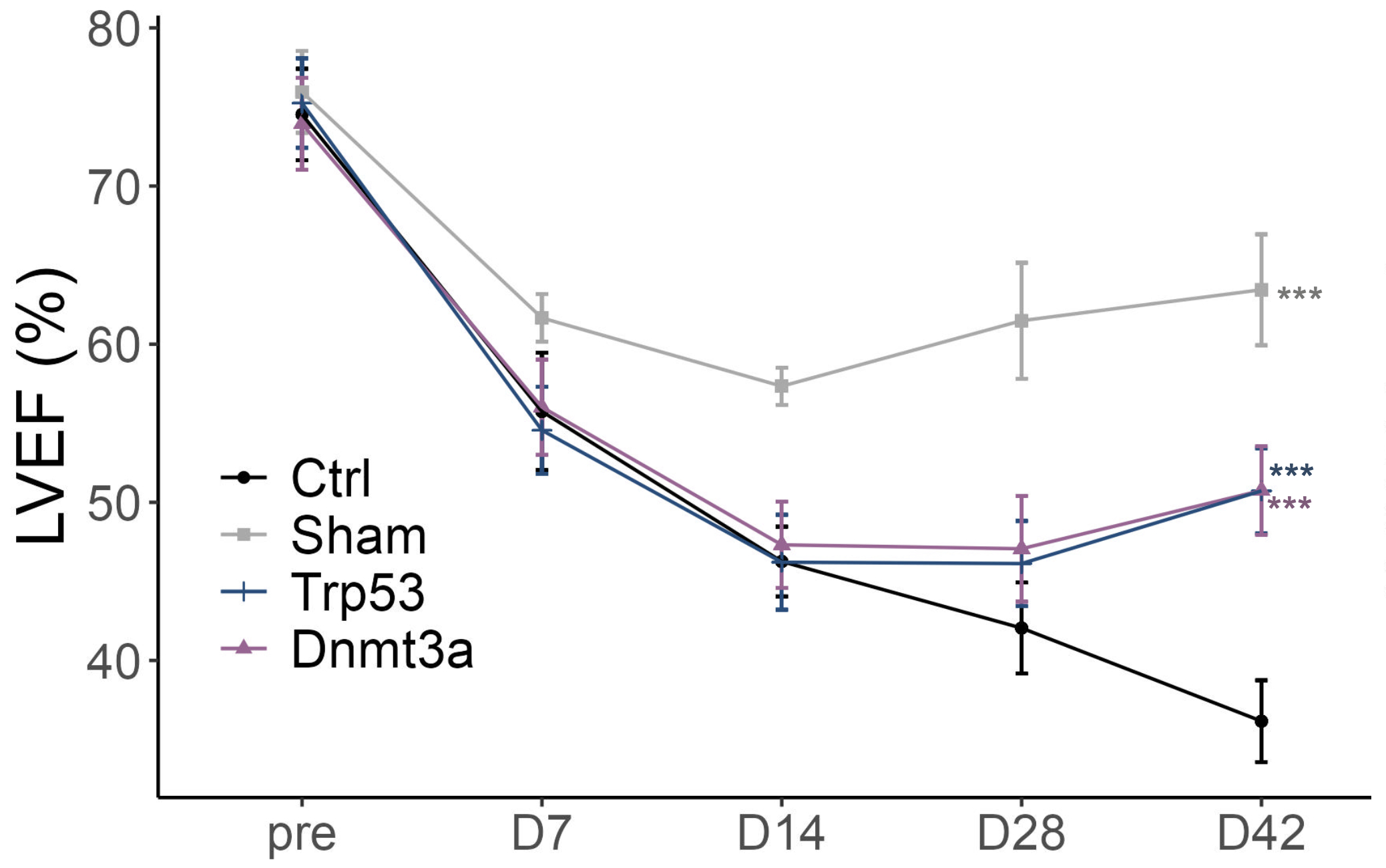
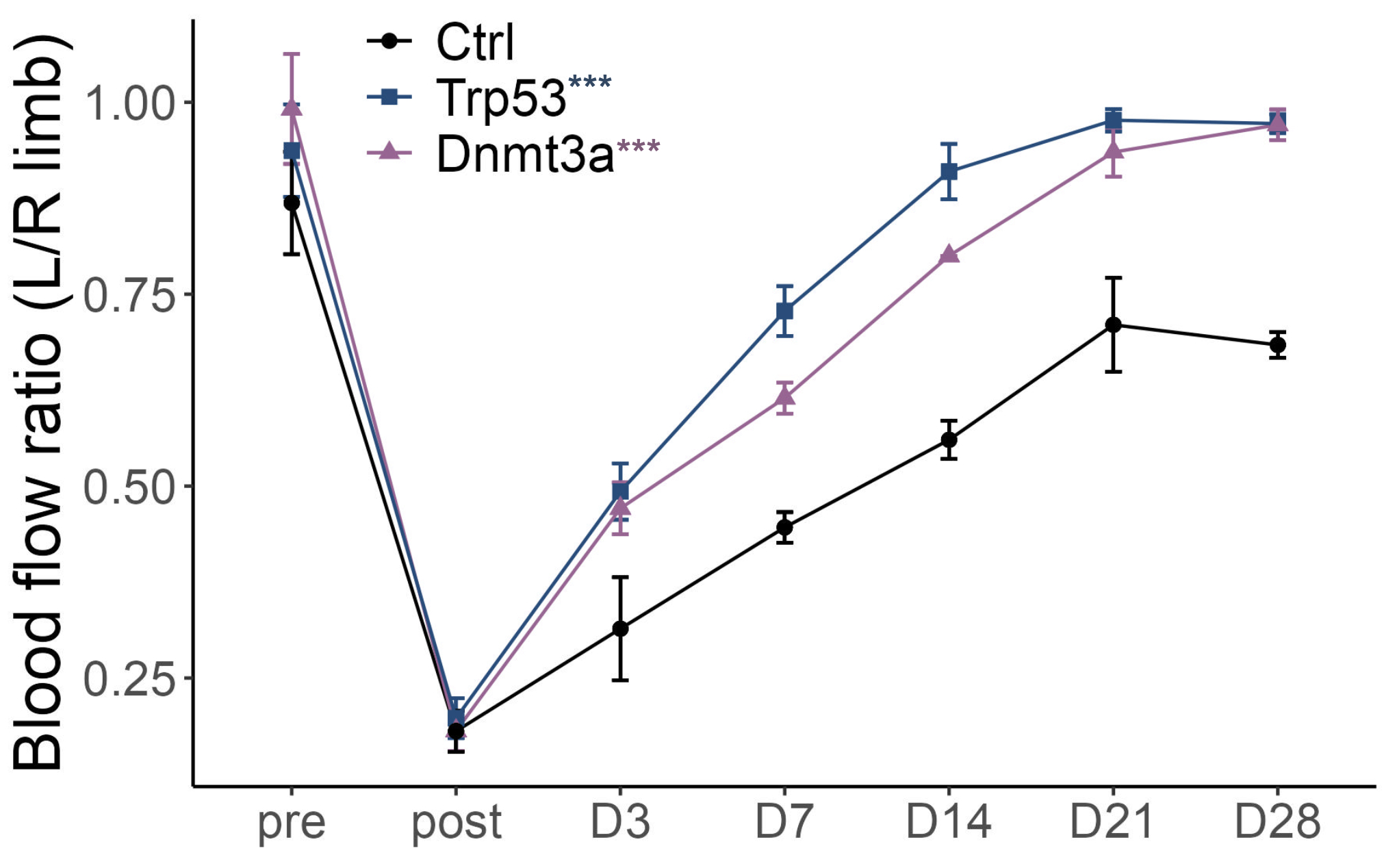
Results: In the UK Biobank cohort, patients with DNMT3A- or TP53-CH had fewer hospital readmissions and improved post-MI survival compared to non-CH individuals. In mouse models, CH enhanced tissue regeneration across MI, HLI, and CTX injuries, as evidenced by improved left ventricular ejection fraction, reduced scar formation, increased neovascularization, and accelerated muscle regeneration. Mechanistically, CH reprogrammed macrophages toward a pro-reparative, angiogenic state. Mutant macrophages exhibited suppressed E2F transcriptional activity and upregulation of Vegfa (VEGF), along with decreased E2F binding and loss of repressive H3K27me3 histone marks at the Vegfa locus. Functional studies confirmed that knockdown of Vegfa or overexpression of E2F in mutant macrophages abolished the reparative benefit.
Conclusions: DNMT3A- and TP53-driven CH promotes cardiac repair following MI through macrophage-mediated angiogenesis and myocyte regeneration. These findings reveal a novel E2F–VEGF axis in mutant macrophages as a key driver of post-ischemic recovery and suggest that engineered macrophages bearing CH-associated mutations may offer a promising therapeutic strategy for ischemic heart and limb disease.
Methods: We analyzed whole-exome sequencing data from the UK Biobank to identify CH-associated mutations in patients with acute CVD and correlated mutation status with clinical outcomes. To model CH in vivo, we transplanted ~10% bone marrow cells harboring Dnmt3a or Trp53 mutations into wild-type mice. Acute CVD models—including myocardial infarction (MI, via left anterior descending artery ligation), hind limb ischemia (HLI, via femoral artery ligation), and cardiotoxin (CTX)-induced muscle injury—were evaluated for functional and histological repair using echocardiography, histology, and fibrosis quantification. Mechanistic studies included single-cell RNA sequencing and epigenetic profiling to uncover pathways contributing to enhanced tissue regeneration.
Results: In the UK Biobank cohort, patients with DNMT3A- or TP53-CH had fewer hospital readmissions and improved post-MI survival compared to non-CH individuals. In mouse models, CH enhanced tissue regeneration across MI, HLI, and CTX injuries, as evidenced by improved left ventricular ejection fraction, reduced scar formation, increased neovascularization, and accelerated muscle regeneration. Mechanistically, CH reprogrammed macrophages toward a pro-reparative, angiogenic state. Mutant macrophages exhibited suppressed E2F transcriptional activity and upregulation of Vegfa (VEGF), along with decreased E2F binding and loss of repressive H3K27me3 histone marks at the Vegfa locus. Functional studies confirmed that knockdown of Vegfa or overexpression of E2F in mutant macrophages abolished the reparative benefit.
Conclusions: DNMT3A- and TP53-driven CH promotes cardiac repair following MI through macrophage-mediated angiogenesis and myocyte regeneration. These findings reveal a novel E2F–VEGF axis in mutant macrophages as a key driver of post-ischemic recovery and suggest that engineered macrophages bearing CH-associated mutations may offer a promising therapeutic strategy for ischemic heart and limb disease.
More abstracts on this topic:
Investigating Transcriptomic Profiles of Peripheral Blood Mononuclear Cells in Moyamoya Disease Using Single-Cell RNAseq
Demirag Zeynep, Uchino Haruto, Tokairin Kikutaro, Rao Shailaja, Chiang Terrance, Morton Gabriella, Lee Alex, Cheng Michelle, Steinberg Gary
Association of Neutrophil-Lymphocyte Ratio With Cardiovascular Mortality and All-cause Mortality in Patients Receiving Chronic Hemodialysis: A Systematic Review and Meta-analysisVempati Roopeessh, Damarlapally Nanush, Vasudevan Srivatsa Surya, Banda Prathibha, Mourad Denise, Polamarasetty Harshavardhan, Mathur Gaurav, Khan Afrasayab, Desai Rupak