Final ID: MP1563
High-fat diet unmasks latent arrhythmogenic remodeling in Mc4r-knockout mice: Insights from a genetic model of obesity-induced atrial fibrillation
Abstract Body (Do not enter title and authors here): Background: While high-fat diet (HFD)-induced obesity is widely used to model obesity-associated atrial fibrillation (AF), the electrophysiological mechanisms in genetic obesity remain poorly defined. Mutations in melanocortin 4 receptor (MC4R) are the most common cause of monogenic obesity in humans. We hypothesized that Mc4r-knockout (Mc4r-KO) mice harbor a latent electrophysiologic substrate that becomes arrhythmogenic upon metabolic stress with HFD, reflecting a two-hit model of AF.
Objective:To investigate how HFD modifies atrial remodeling and AF susceptibility in Mc4r-KO mice and to delineate shared and divergent mechanisms relative to standard diet-fed Mc4r-KO and diet-induced obesity (DIO) models.
Methods: Mc4r-KO mice were fed either standard chow (MS) or a 60% HFD (DIO Mc4r-KO or MD) for 10 weeks and compared with wild-type (WT) controls. AF burden was assessed using transesophageal pacing. Atrial electrophysiology and remodeling were evaluated by patch clamping, echocardiography, mitoSOX staining, and transcriptomics.
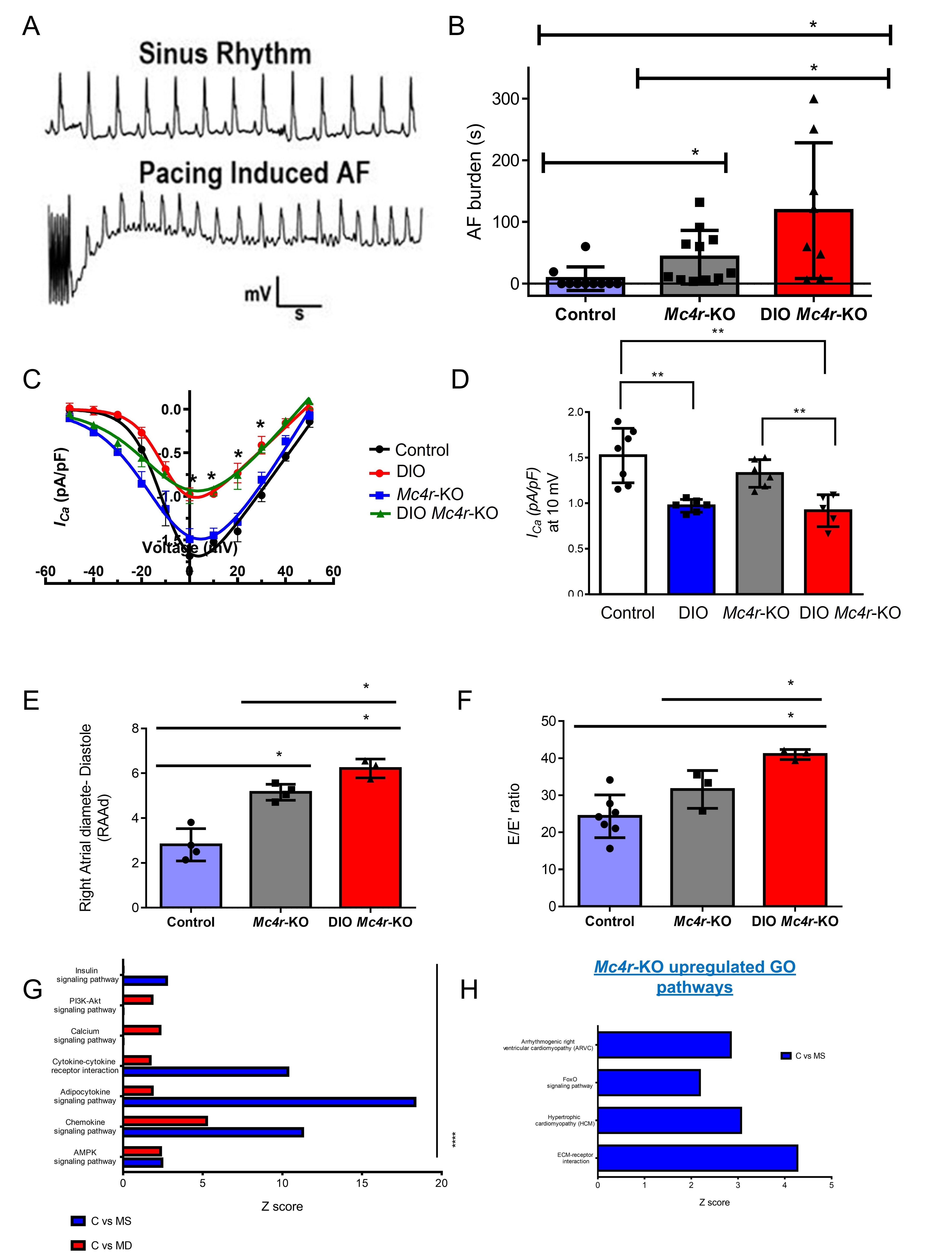
Results: DIO-Mc4r-KO (MD) mice exhibited a marked increase in AF burden compared to chow-fed Mc4r-KO (MS) mice, despite similar body weights (Figure 1A-b). This increase was associated with a significant reduction in atrial L-type calcium current (ICa,L), whereas MS mice showed didnt (Figure 1C-D). Echocardiography showed elevated E/E′ ratios and right atrial dilation in both groups, but LV mass increased only in MD mice (Figure 1E-F). Transcriptomic analysis revealed no overlap in upregulated cardiac pathways between MD and MS mice. MS hearts exhibited upregulation of adaptive metabolic and structural pathways (oxidative phosphorylation, MAPK signaling, ECM-receptor interaction), consistent with preserved remodeling. Downregulation of calcium signaling and PI3K-Akt pathways in MD mice further confirmed maladaptive electrophysiological remodeling (Figure 1G-H).
Conclusion: MC4R deficiency alone causes mild atrial changes without significant AF susceptibility, but exposure to a HFD unmasks a profound electrophysiologic and metabolic vulnerability. This two-hit model highlights how genetic obesity combined with metabolic stress drives arrhythmogenic remodeling, offering new insights into AF mechanisms and potential therapeutic targets.
Objective:To investigate how HFD modifies atrial remodeling and AF susceptibility in Mc4r-KO mice and to delineate shared and divergent mechanisms relative to standard diet-fed Mc4r-KO and diet-induced obesity (DIO) models.
Methods: Mc4r-KO mice were fed either standard chow (MS) or a 60% HFD (DIO Mc4r-KO or MD) for 10 weeks and compared with wild-type (WT) controls. AF burden was assessed using transesophageal pacing. Atrial electrophysiology and remodeling were evaluated by patch clamping, echocardiography, mitoSOX staining, and transcriptomics.
Results: DIO-Mc4r-KO (MD) mice exhibited a marked increase in AF burden compared to chow-fed Mc4r-KO (MS) mice, despite similar body weights (Figure 1A-b). This increase was associated with a significant reduction in atrial L-type calcium current (ICa,L), whereas MS mice showed didnt (Figure 1C-D). Echocardiography showed elevated E/E′ ratios and right atrial dilation in both groups, but LV mass increased only in MD mice (Figure 1E-F). Transcriptomic analysis revealed no overlap in upregulated cardiac pathways between MD and MS mice. MS hearts exhibited upregulation of adaptive metabolic and structural pathways (oxidative phosphorylation, MAPK signaling, ECM-receptor interaction), consistent with preserved remodeling. Downregulation of calcium signaling and PI3K-Akt pathways in MD mice further confirmed maladaptive electrophysiological remodeling (Figure 1G-H).
Conclusion: MC4R deficiency alone causes mild atrial changes without significant AF susceptibility, but exposure to a HFD unmasks a profound electrophysiologic and metabolic vulnerability. This two-hit model highlights how genetic obesity combined with metabolic stress drives arrhythmogenic remodeling, offering new insights into AF mechanisms and potential therapeutic targets.