Final ID: Mo4042
Study on the Mechanism of Protocatechualdehyde in Improving Sepsis-Induced Cardiomyopathy by Targeting Mitochondrial ROS and Inhibiting the GSDMD/STING Pathway in Macrophage
Abstract Body (Do not enter title and authors here): Background:
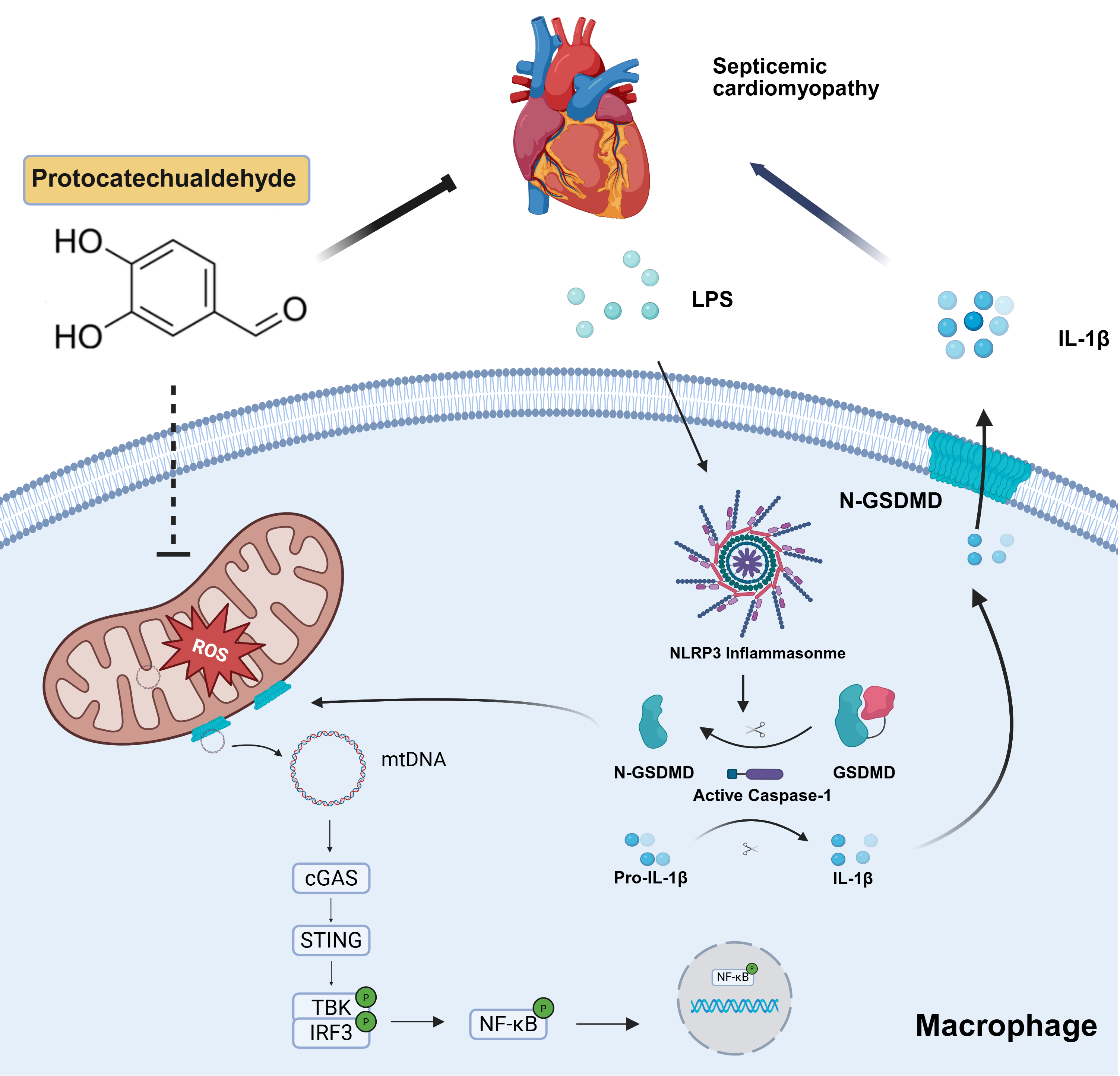
Sepsis-induced cardiomyopathy (SICM) is a major cause of high morbidity and mortality in septic patients. In SICM, macrophage infiltration and aberrant immune activation play a critical role in triggering inflammatory responses in cardiac tissue. Our previous studies identified that among 23 natural small molecules, Protocatechualdehyde (PCA) exhibited the most potent inhibitory effect on macrophage inflammation. However, the effects of PCA on sepsis-induced cardiac dysfunction remain poorly understood.
Research Questions:
This study aims to investigate the role of the small molecule PCA in sepsis-induced cardiomyopathy and the underlying potential mechanisms involved.
Methods:
We established a sepsis mouse model using cecal ligation and puncture (CLP) and treated the mice with intraperitoneal injections of 20 mg/kg and 40 mg/kg of PCA for 5 consecutive days. Heart function was evaluated by measuring survival time, heart function biomarkers, and hemodynamic parameters. Lipopolysaccharide (LPS)-treated mouse bone marrow-derived macrophages (BMDMs) were used to establish a macrophage pyroptosis model. We assessed the activation of NLRP3 inflammasomes, the release of inflammatory cytokines, and gasdermin-D (GSDMD)-mediated mitochondrial pore formation and mitochondrial DNA leakage, and examined their effects on downstream STING/IRF3 signaling pathways.
Rusult:
The in vivo results of this study demonstrated that PCA significantly alleviated cardiac dysfunction, inflammatory cell infiltration, and the production of inflammatory cytokines in septic mice. Further in vitro experiments showed that PCA inhibited the activation of NLRP3 inflammasome in BMDMs from mice and reduced GSDMD-mediated pyroptosis as well as the activation of the downstream STING/IRF3 pro-inflammatory pathway. Mechanistically, PCA reduced the production of mitochondrial reactive oxygen species (mtROS), thereby inhibiting the activation of the NLRP3 inflammasome and the formation of N-GSDMD. This, in turn, reduced the accumulation of N-GSDMD on both the cell membrane and mitochondrial membrane, further inhibiting the release of mitochondrial DNA (mtDNA) into the cytoplasm. Ultimately, this suppressed the activation of the downstream STING/IRF3 pro-inflammatory pathway, leading to a reduction in the release of inflammatory cytokines.
Conclusion:
These results highlight the therapeutic role of PCA in the resolution of sepsis-induced cardiac inflammation.
Sepsis-induced cardiomyopathy (SICM) is a major cause of high morbidity and mortality in septic patients. In SICM, macrophage infiltration and aberrant immune activation play a critical role in triggering inflammatory responses in cardiac tissue. Our previous studies identified that among 23 natural small molecules, Protocatechualdehyde (PCA) exhibited the most potent inhibitory effect on macrophage inflammation. However, the effects of PCA on sepsis-induced cardiac dysfunction remain poorly understood.
Research Questions:
This study aims to investigate the role of the small molecule PCA in sepsis-induced cardiomyopathy and the underlying potential mechanisms involved.
Methods:
We established a sepsis mouse model using cecal ligation and puncture (CLP) and treated the mice with intraperitoneal injections of 20 mg/kg and 40 mg/kg of PCA for 5 consecutive days. Heart function was evaluated by measuring survival time, heart function biomarkers, and hemodynamic parameters. Lipopolysaccharide (LPS)-treated mouse bone marrow-derived macrophages (BMDMs) were used to establish a macrophage pyroptosis model. We assessed the activation of NLRP3 inflammasomes, the release of inflammatory cytokines, and gasdermin-D (GSDMD)-mediated mitochondrial pore formation and mitochondrial DNA leakage, and examined their effects on downstream STING/IRF3 signaling pathways.
Rusult:
The in vivo results of this study demonstrated that PCA significantly alleviated cardiac dysfunction, inflammatory cell infiltration, and the production of inflammatory cytokines in septic mice. Further in vitro experiments showed that PCA inhibited the activation of NLRP3 inflammasome in BMDMs from mice and reduced GSDMD-mediated pyroptosis as well as the activation of the downstream STING/IRF3 pro-inflammatory pathway. Mechanistically, PCA reduced the production of mitochondrial reactive oxygen species (mtROS), thereby inhibiting the activation of the NLRP3 inflammasome and the formation of N-GSDMD. This, in turn, reduced the accumulation of N-GSDMD on both the cell membrane and mitochondrial membrane, further inhibiting the release of mitochondrial DNA (mtDNA) into the cytoplasm. Ultimately, this suppressed the activation of the downstream STING/IRF3 pro-inflammatory pathway, leading to a reduction in the release of inflammatory cytokines.
Conclusion:
These results highlight the therapeutic role of PCA in the resolution of sepsis-induced cardiac inflammation.
More abstracts on this topic:
A mechanism whereby SGLT2 inhibitor dapagliflozin reverses cardiac diastolic dysfunction in a model of HFpEF
Liu Man, Liu Hong, Kang Gyeoung-jin, Kim Eunji, Neumann Mitchell, Johnson Madeline, Murikinati Ruthvika, Dudley Samuel
A Curious Complete Heart Block with CarfilzomibShah Mohammed, Rahman Naveed, Al-mohamad Talal, Batra Sejal, Vyas Apurva