Final ID: MP2384
Efficacy of Bempedoic Acid Versus Placebo for Additional Lipid Lowering in Patients Receiving Background Statin Therapy: A Meta-Analysis of Randomized Controlled Trials
Bempedoic acid, an oral ATP-citrate lyase inhibitor, is indicated as an adjunct to maximally tolerated statin therapy for patients with atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH) who require additional LDL-C lowering. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend bempedoic acid as a nonstatin option for further LDL-C reduction in these populations, particularly when statin therapy alone is insufficient or not tolerated.
Methodology
A systematic search of databases including PubMed, Google Scholar, ScienceDirect, Cochrane Library, and clinicaltrials.gov was conducted through May 2025. We included randomized trials comparing bempedoic acid plus maximally tolerated statin therapy with placebo plus background statin, reporting three key outcomes, including changes in non–HDL cholesterol, total cholesterol, or apolipoprotein B at 12 weeks, in patients with established atherosclerotic cardiovascular disease (ASCVD) and heterozygous familial hypercholesterolemia (HeFH). A meta-analysis was performed in RevMan 5.4.1 using a random-effects model to assess heterogeneity.
Results
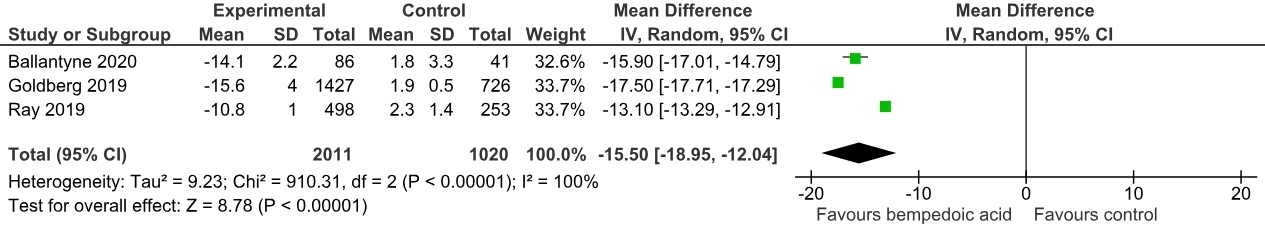
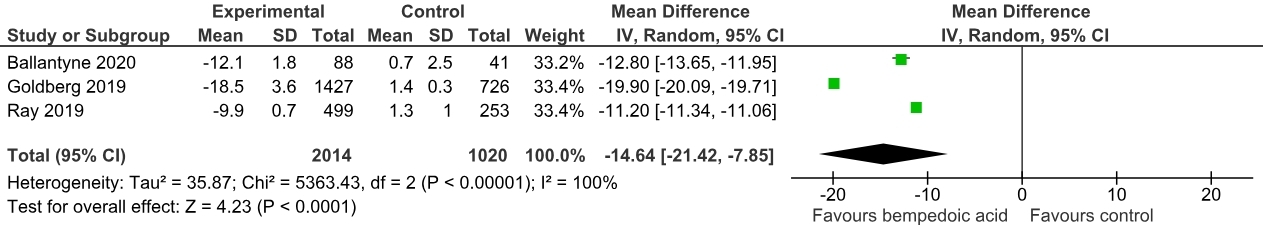
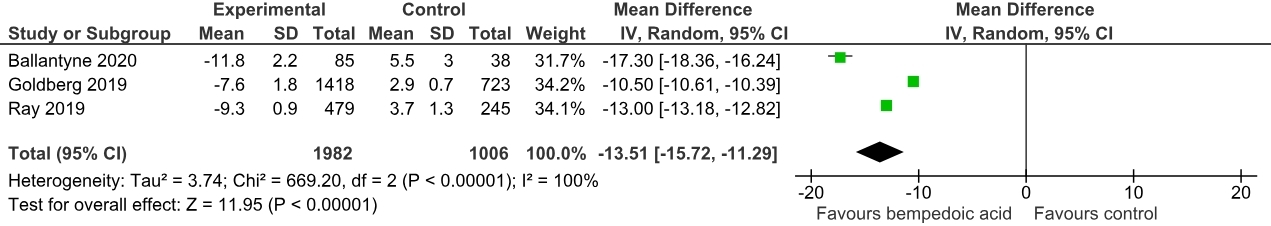
Three randomized controlled trials with a total of 3034 patients were included, comprising 2014 cases (mean age: 65.3 ± 9.1, male: 69.9%) and 1020 controls (mean age: 66.2 ± 8.8, male: 69.3%). A pooled analysis showed a significant non-HDL-C reduction in the Bempedoic acid group compared to the placebo [Mean difference (MD): -15.50 mg/dL; 95% CI, -18.95 to -12.04; p<0.0001]. Similarly, a significant reduction in total cholesterol levels (MD: -14.64 mg/dL; 95% CI, -21.42 to -7.85; p<0.00001) and apolipoprotein B levels (MD: -13.51 mg/dL; 95% CI, -15.72 to -11.29; p<0.00001) was noted.
Conclusion
The addition of bempedoic acid compared with placebo resulted in a significant lowering of various cholesterol subfraction levels over 12 weeks. Our meta-analysis reiterates the efficacy of bempedoic acid as lipid-lowering therapy in patients with established ASCVD & HeFH. The ES-BempedACS trial is an ongoing trial that further compares the outcomes of bempedoic acid use in combination with statins in a large sample size. There is a need for large-scale RCTs to establish the inclusion of Bempedoic acid in guideline-directed lipid-lowering therapy.
- Singla, Ankur ( Northwest Health-Porter, Valparaiso , Valparaiso , India )
- Syed, Saif ( RCSI , Dublin , Ireland )
- Thummala, Sumaina ( NYCHHC- Woodhull Medical Centre , Brooklyn , New York , United States )
- Grover, Jahanvi ( Pt. BDS Institute of Health Sciences , Rohtak , India )
- Mahendru, Diksha ( Main line Health, Lankenau Medical Center , Bryn Mawr , Pennsylvania , United States )
- Aziz, Imran ( Cook County Health , Chicago , Illinois , United States )
- Philip, Anil ( John H Stroger of Cook County , Chicago , Illinois , United States )
- George, Lina ( Cook County Health, John H Stroger , Chicago , Illinois , United States )
- Mautong, Hans ( John H. Stroger Jr. Hospital , Chicago , Illinois , United States )
- Chaturvedi, Abhishek ( Virginia Commonwealth University , Richmond , Virginia , United States )
- Saha, Shubhashis ( Cook County Health, John H Stroger , Chicago , Illinois , United States )
- Brar, Ajit ( Hurley Medical Center , Auburn hills , Michigan , United States )
- Sodhi, Sohail Singh ( Trinitas Regional Medical Center , Elizabeth , New Jersey , United States )
- Garg, Ayushi ( Trident Medical Center , North Charleston , South Carolina , United States )
- Bansal, Nahush ( University of Toledo , Toledo , Ohio , United States )
- Bhatia, Hitesh ( Guthrie Robert Packer Hospital , Sayre , Pennsylvania , United States )
- Jain, Hritvik ( AIIMS Jodhpur , Jodhpur , India )
- Ravi, Soumiya ( University of Arizona , Tucson , Arizona , United States )
Meeting Info:
Session Info:
Closing the LDL Gap: Innovations, Access, and Adherence in Lipid-Lowering Therapy
Monday, 11/10/2025 , 01:45PM - 02:55PM
Moderated Digital Poster Session
More abstracts on this topic:
Luebbe Samuel, Wilkins John, Moran Andrew, Sniderman Allan, Kohli-lynch Ciaran
Adherence to Lipid Lowering Therapy among US Veterans with Atherosclerotic Cardiovascular DiseaseWard Rachel, Gaziano Michael, Wellman Helen, Yel Nedim, Young Melissa, Coleman-lopez Mason, Niu Xiaoli, Mcelligott Sean, Gagnon David, Djousse Luc
More abstracts from these authors:
Difference in Characteristics and Outcomes of Atrial Fibrillation patients based on type of Heart Failure: An NRD Propensity Matched Analysis
Brar Ajit, Ravi Soumiya, Garg Ayushi, Chirumamilla Yashitha, Omer Mohammed, Maharjan Nikky, Maini Shriya, Alkotob Luay
Characteristics and Association of Cannabis Use on Cardiovascular Outcomes in patients hospitalized with Atrial Fibrillation: An NRD Propensity Matched AnalysisBrar Ajit, Ravi Soumiya, Chirumamilla Yashitha, Garg Ayushi, Omer Mohammed, Maharjan Nikky, Maini Shriya, Zreik Ali