Final ID: Mo3106
Early Left Ventricular Reverse Remodeling and Predictors of All-Cause Mortality after Successful Transcatheter Aortic Valve Replacement for Severe Symptomatic Aortic Stenosis
Abstract Body (Do not enter title and authors here): Background. In patients with aortic stenosis (AS), left ventricular (LV) remodeling is a complex process involving both structural and functional abnormalities, due to increased chronic pressure afterload. Evidence remains limited regarding early LV reverse remodeling post transcatheter aortic valve replacement (TAVR), and its potential prognostic significance.
Objectives. To assess (1) early LV reverse remodeling after TAVR; (2) predictors associated with reverse remodeling; (3) independent parameters associated with all-cause mortality.
Methods. Transthoracic echocardiography was performed prior to TAVR and before discharge (5±4 days), in pts with severe symptomatic AS who underwent successful TAVR. Early LV reverse remodeling was assess by changes in LV ejection fraction (LVEF), interventricular septum wall (ISW) and posterior wall (PW) thickness, LV mass and diastolic function (E/A, left atrial diameter).
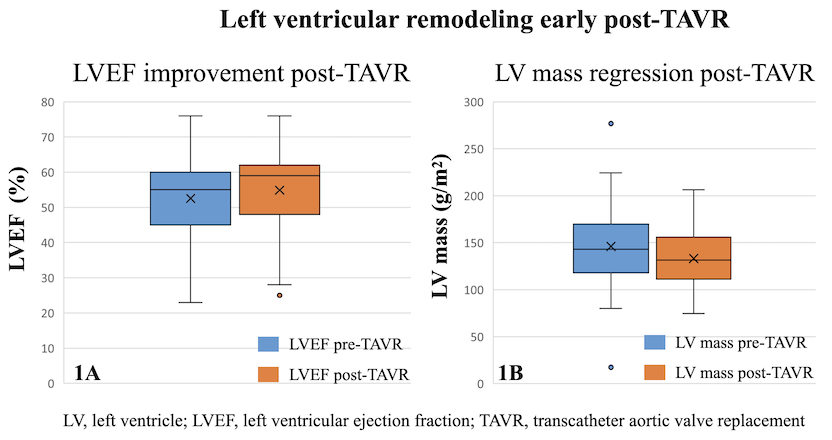
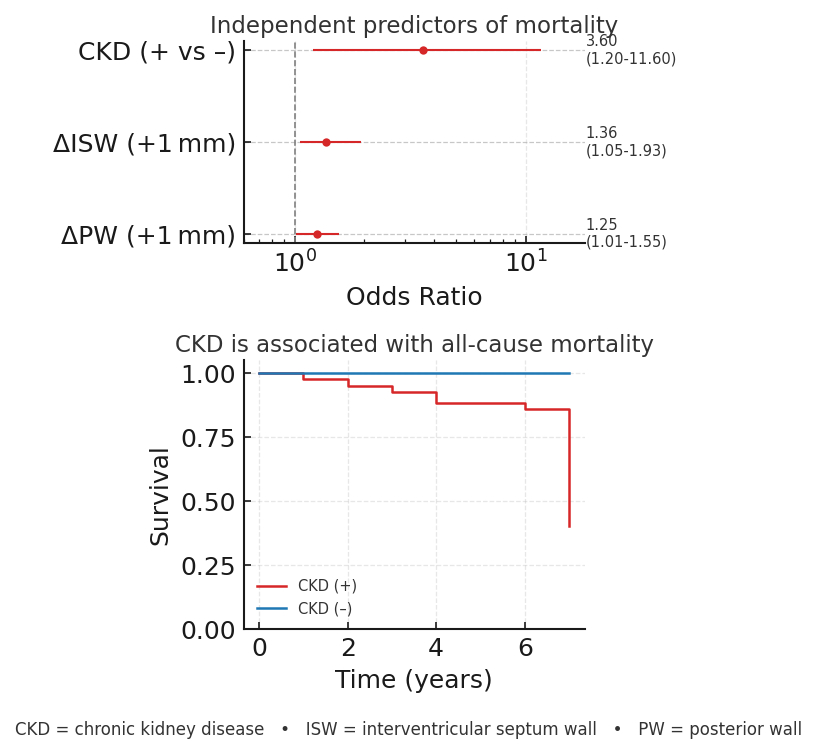
Results. A total of 147 pts were included (age 78±5 yrs, 51% women). Over a median follow-up of 4 yrs (IQR 2 to 6 yrs), 12 pts (8%) died. Early post-TAVR there was significant improvement in LVEF (Image 1A) and regression of LV mass (Image 1B), with no significant early changes in diastolic parameters. Additionally, patients with chronic kidney disease (CKD) showed greater increase in LVEF (t=2.3, p=0.024), while those with insulin-dependent diabetes showed greater regression in LV mass (t=2.5, p=0.012). Patients admitted in NYHA classes III-IV (73 pts, 50%) had greater improvement in both LVEF (t=-3.7, p<0.001) and LV mass (t=2.5, p=0.011). Inadequate regression of ISW thickness was the strongest echocardiographic predictor of death (t=3.51, p=0.001); a lower, but significant effect was seen for PW (t=2.20, p=0.034). On multivariable analysis, CKD was the only independent parameter associated with all-cause mortality (OR=3.6, p=0.018, AUC=0.86)(Image 2).
Conclusions. Patients with severe symptomatic AS experience LV reverse remodeling early after successful TAVR, as demonstrated by significant improvement in LVEF and regression of LV hypertrophy, especially in those with associated comorbidities (more advanced heart failure, insulin-dependent diabetes, CKD). Limited regression of LV hypertrophy and CKD were the strongest predictors of death.
Objectives. To assess (1) early LV reverse remodeling after TAVR; (2) predictors associated with reverse remodeling; (3) independent parameters associated with all-cause mortality.
Methods. Transthoracic echocardiography was performed prior to TAVR and before discharge (5±4 days), in pts with severe symptomatic AS who underwent successful TAVR. Early LV reverse remodeling was assess by changes in LV ejection fraction (LVEF), interventricular septum wall (ISW) and posterior wall (PW) thickness, LV mass and diastolic function (E/A, left atrial diameter).
Results. A total of 147 pts were included (age 78±5 yrs, 51% women). Over a median follow-up of 4 yrs (IQR 2 to 6 yrs), 12 pts (8%) died. Early post-TAVR there was significant improvement in LVEF (Image 1A) and regression of LV mass (Image 1B), with no significant early changes in diastolic parameters. Additionally, patients with chronic kidney disease (CKD) showed greater increase in LVEF (t=2.3, p=0.024), while those with insulin-dependent diabetes showed greater regression in LV mass (t=2.5, p=0.012). Patients admitted in NYHA classes III-IV (73 pts, 50%) had greater improvement in both LVEF (t=-3.7, p<0.001) and LV mass (t=2.5, p=0.011). Inadequate regression of ISW thickness was the strongest echocardiographic predictor of death (t=3.51, p=0.001); a lower, but significant effect was seen for PW (t=2.20, p=0.034). On multivariable analysis, CKD was the only independent parameter associated with all-cause mortality (OR=3.6, p=0.018, AUC=0.86)(Image 2).
Conclusions. Patients with severe symptomatic AS experience LV reverse remodeling early after successful TAVR, as demonstrated by significant improvement in LVEF and regression of LV hypertrophy, especially in those with associated comorbidities (more advanced heart failure, insulin-dependent diabetes, CKD). Limited regression of LV hypertrophy and CKD were the strongest predictors of death.
More abstracts on this topic:
Aortic Root Pressure for Detecting Aortic Stenosis using Machine Learning
Dunn Michael, Lalush David, Wheaten Sterling, Stouffer George, Syed Faisal
Acoustic Analysis For The Detection Of Leaflet Thrombosis After Transcatheter Aortic Valve ReplacementLupu Lior, Shetrit Aviel, Kadosh Adi, Aviram Galit, Topilsky Yan, Banai Shmuel, Havakuk Ofer